Untangling the Complexities of Cell and Gene Therapy Clinical Trials: A Supply Chain Perspective
By Luisa Sterkel & Joana Loureiro, Tenthpin Consultants The promise and potential of cell and gene therapies (CGT) has emerged…
By Luisa Sterkel & Joana Loureiro, Tenthpin Consultants
The promise and potential of cell and gene therapies (CGT) has emerged in the recent past and currently over 1.500 CGT are registered for clinical trials holding great hope for the treatment of challenging and uncurable diseases. The ability to modify or introduce genetic material in human cells in such a precise and patient-centered manner clearly constitutes a breakthrough in personalized medicine. Every major pharma company is now involved in CGT development which has resulted in the approval of 28 therapies by the FDA thereby making CGT no longer a niche category of therapies. This promise and growth are expected to continue as CGT, which have the potential to cover 70% of oncology drugs, are being developed to treat further disease types such as solid tumors.
CGT drugs fall into two major categories: autologous and allogeneic. While both begin with human biological material then seek to edit the cellular genetics and grow the cell population for later transfusion or transplant back into the patient, these two drug categories differ in one highly impactful way affecting the clinical trial supply chain.
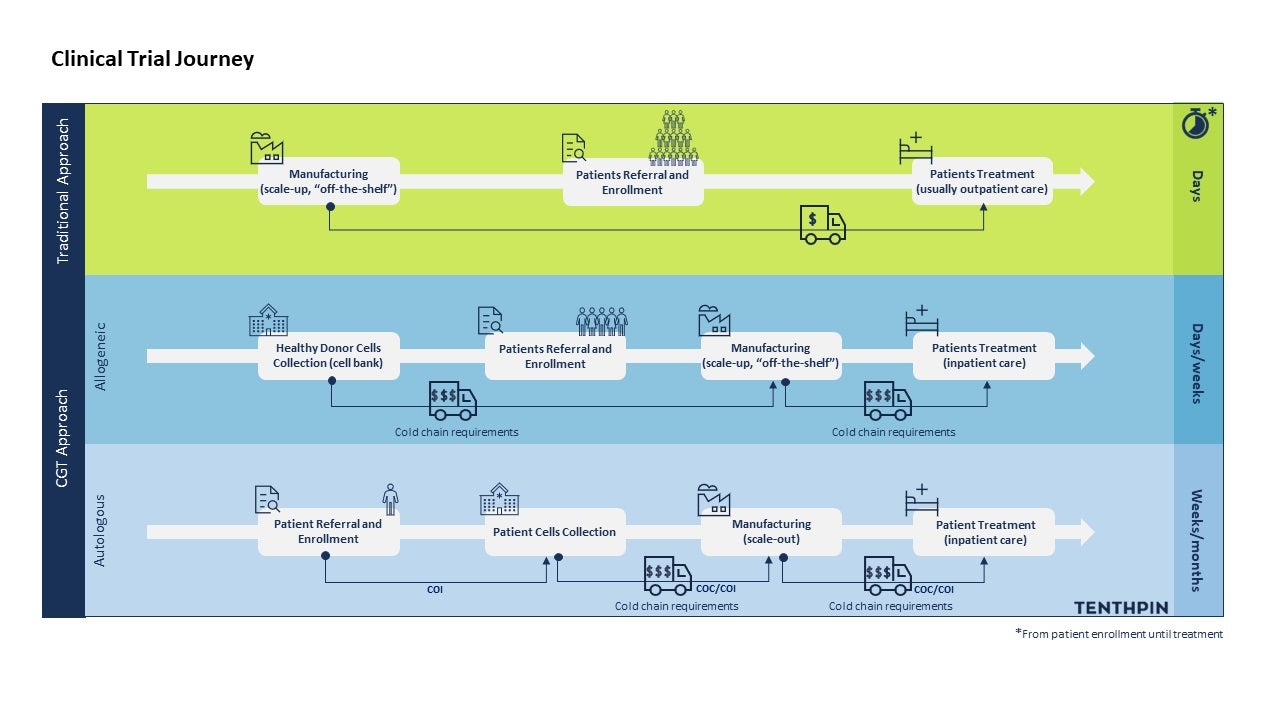
Autologous therapies are characterized as a circular supply chain; the patient’s own sample starts the supply chain, and it is then subjected to ex-vivo modifications with the resulting therapy administered back to that same patient. Allogeneic therapies begin with healthy donor samples to develop the eventual therapeutic product which can be administered to multiple patients. This major difference leads to very different requirements and processes for the clinical trial supply chain, which are summarized below.
What are the general differences in the supply chain of CGT vs. traditional clinical trials?
Nearly every biopharma with a portfolio of drugs in development spanning traditional small molecules and biologics and the newer CGT operates with separate clinical supply organizations per these therapy areas. This is due to major differences throughout the clinical supply chain in both requirements and management practices. Below there are listed common differences across both the autologous and allogeneic CGT versus the traditional drug clinical supply chain:
1. CGT trials operate with much lower volumes of manufactured final product. This is owed to two primary factors:
- CGT trials have fewer patients (broadly applied, on average 10-40% of the patient volumes of traditional trials). This is due to the advanced disease stage of the patients for these often lifesaving therapies as well as the typical rare disease conditions they seek to treat.
- Most often the CGT patient dose administration is a single dose or limited periodic dose.
2. CGT have highly unique and specialized manufacturing processes. This is a primary factor for the staggeringly high unit cost of CGT.
3. Cold chain requirements for CGT are highly stringent and most often at the cryogenic level (below -150 Celsius), which requires the use of specialized containers filled with liquid nitrogen (dewars) and particular shippers requiring specific logistics expertise.
4. As biologic products, CGT have extremely low shelf life (SL), and typically must be administered in days, except when they are cryopreserved and shipped in cryogenic conditions. This typically adds 3x more costs with oversight, storage, and transportation than traditional therapies.
5. Patients need to pass through a conditioning period to be clinically prepared to receive the therapy. This implies precise (and frequently changing) timeline planning for drug manufacture and delivery.

Differences in CGT vs traditional trials supply chain. Time needed for manufacturing steps could vary depending on the procedures involved and, on the possibility, to cryopreserve (or not) the final product to make it “off the shelf” (for allogeneic). Furthermore, the eventual need for a multiple dose treatment will influence this range of time.
Specific differences in the allogeneic CGT supply chain
Accepting the above listed common differences in the supply chains between CGT and traditional trials, it is perhaps surprising to learn that allogeneic therapy trials are highly comparable to traditional clinical trials for their supply chain requirements and processes. In fact, from a clinical supply chain process map perspective, the changes are few however highly impactful:
1. The labeling process of the final product is earlier than that for traditional trials.
2. Clinical supply chain planning needs to account for shorter therapy SL and more stringent temperature management. The impact of the shorter SL is experienced most per the logistics timing requirements:
- For inter-country shipments, export/import management needs to operate in a proactive manner as procedures, regulations and timings at country level often do not meet CGT realities.
- 3rd party air and local transport conditions require upfront contingency planning at the shipments level, often then monitored and managed at the specific patient shipment by triage teams and rapid decision processes (I.e., plan a, plan b, … charter flight standby option).
Major differences in the autologous CGT supply chain
The vein-to-vein fundamental nature of autologous therapies and inability to operate at scale beyond the individual patient sample through dose delivery process leads to further significant differences in the clinical trials supply chain and logistics requirements. And here the clinical supply chain processes for the autologous clinical trials are very different per traditional drug therapies as they are designed and adjusted frequently per the patient’s individual needs. Below we point out the major differences:
1. Due to the patient sample collection, manipulation and delivery to that same patient, additional tracking, and controls to ensure that patient match is maintained through the supply chain and the supporting logistics process must be in place:
- A chain of identity (COI) must be established at the point of the patient sample (on the sample label) and adhered through the manufacturing and logistics process up to the therapy delivery.
- A chain of custody (COC) must also be tracked and recorded as the sample and later the final product is moved between parties.
2. Time for both the patient and the therapy in terms of readiness and viability are carefully monitored – autologous therapies need to be available and delivered in a narrow window (typically in a single day). Patient level scheduling is crucial for the treatment success: the product should be delivered during the patient readiness window for infusion; the product quality must be ensured (consider SL); and logistic companies must manage their timely delivery of cryogenics (deliver samples in dewars and collection of these containers at that time). Changes in the patient’s clinical parameters or in the manufacturing, batch results review and release, and logistics processes must be monitored proactively by pre-set triage teams to determine swift logistics reactions.
3. Combining the circular supply chain nature of these therapies and the unique and cutting-edge drug manufacturing processes with the additional tracking requirements and proactive triage focused manufacturing through to therapy delivery leads to far greater resource intensity for just a single per patient shipment. Taken together, this all leads to eye-popping costs for the drug sponsor and of course for the patient and relevant insurance provider.
Key takeaways for the road ahead
Global incidence rates of cancer and rare diseases are expected to continue increasing, and more sophisticated diagnostic methods are contributing to these numbers. However, unfortunately, there is still no cure for a huge number of these pathologies. This drives the exponential growth of CGT as a personalized treatment option, offering new hope for millions of patients. Nonetheless, CGT raises different concerns that have direct impact on how the pharmaceutical industry has traditionally managed clinical trials.
The purpose of this article is to list major differences that are ‘disruptive’ to the current clinical trial supply chain, elucidating the R&D community for the complexities that can be encountered. In a nutshell, CGT trials bring challenges from manufacturing to the final product delivery, including smaller quantities, more complex drug manufacturing, higher costs, specific regulatory requirements, and more complex distribution and storage.
Significant investments have been made in this field, therefore future promise and potential dominance of CGT will play out over the next 10 plus years. It is important to recognize that the history is short for CGT trials and improvements in processes as well as capabilities will be forthcoming. To date, the focus on CGT has been on orchestration solutions, mainly for autologous therapies.
The key to successful CGT trials lies in specialized software, trained staff, collaborations with experts, and robust facilities, which collectively will make these products more affordable. As such, life science enterprises should seek to be well networked and continually ask themselves if they have the know-how, facilities, and technology to double down on CGT trials.
What's Your Reaction?