Understanding the cellular mechanisms of obesity-induced inflammation and metabolic dysfunction
A research team led by Professor Jong Kyoung Kim and Yujin Jeong (PhD candidate), from the Department of Life Sciences at Pohang University of Science and Technology (POSTECH) in collaboration with Professor Yun-Hee Lee and Cheoljun Choi (PhD candidate) from the College of Pharmacy at Seoul National University, Professor Young-Min Hyun and Koung-Min Park (PhD […]
A research team led by Professor Jong Kyoung Kim and Yujin Jeong (PhD candidate), from the Department of Life Sciences at Pohang University of Science and Technology (POSTECH) in collaboration with Professor Yun-Hee Lee and Cheoljun Choi (PhD candidate) from the College of Pharmacy at Seoul National University, Professor Young-Min Hyun and Koung-Min Park (PhD candidate) from Yonsei University College of Medicine, Professor James Granneman from Wayne State University (WSU), and Professor Young-Suk Jung from the College of Pharmacy at Pusan National University, spearheaded a research endeavor that successfully uncovered the mechanisms governing inflammation and metabolic dysfunction in tissues associated with obesity. Their findings were recently published in the international journal Nature Communications.

Credit: POSTECH
A research team led by Professor Jong Kyoung Kim and Yujin Jeong (PhD candidate), from the Department of Life Sciences at Pohang University of Science and Technology (POSTECH) in collaboration with Professor Yun-Hee Lee and Cheoljun Choi (PhD candidate) from the College of Pharmacy at Seoul National University, Professor Young-Min Hyun and Koung-Min Park (PhD candidate) from Yonsei University College of Medicine, Professor James Granneman from Wayne State University (WSU), and Professor Young-Suk Jung from the College of Pharmacy at Pusan National University, spearheaded a research endeavor that successfully uncovered the mechanisms governing inflammation and metabolic dysfunction in tissues associated with obesity. Their findings were recently published in the international journal Nature Communications.
According to the World Health Organization (WHO), approximately 16% of the global population is obese as of 2022. This epidemic represents one of the most rapidly escalating diseases worldwide and is serving as a leading cause of various metabolic disorders such as diabetes, hypertension, and atherosclerosis.
Overconsumption of nutrients prompts the infiltration of diverse types of macrophages into adipose tissue. Among them, certain macrophages play a role in clearing deceased cells and upholding tissue balance while others elicit inflammatory reactions. In patients with obesity, the population of these inflammatory macrophages escalates swiftly, exacerbating issues related to inflammation and metabolic function.
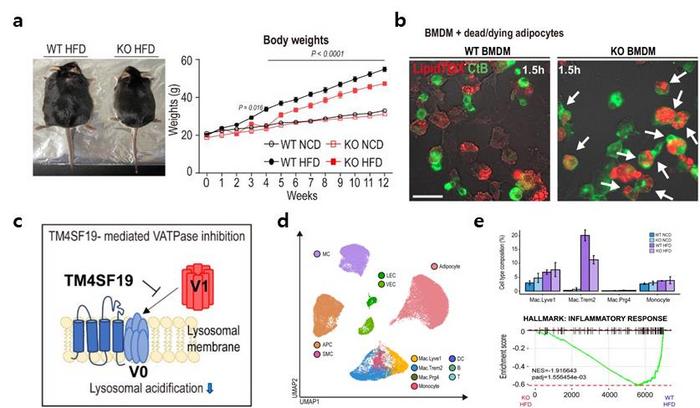
Employing animal trials, single-nucleus RNA sequencing, and intravital imaging techniques, the research team scrutinized TM4SF19, a protein specifically present in inflammatory macrophages.
The findings revealed a notable increase in TM4SF19 levels within the adipose tissue of animal subjects subjected to a high-fat diet. Remarkably, the researchers unveiled that this protein inhibits a pump (V-ATPase) present in lysosomes, which harbor various hydrolytic enzymes and play a crucial role in lysosomal pH regulation. Consequently, this impedes the phagocytic process through which macrophages eliminate spent adipocytes.
Conversely, macrophages deficient in TM4SF19 demonstrated significantly enhanced efficacy in clearing deceased adipocytes. This not only deterred weight gain induced by a high-fat diet but also improved metabolic dysfunction by curbing tissue inflammation and insulin resistance. The significance of this research lies in its identification of TM4SF19, present on inflammatory macrophages, as pivotal in mitigating inflammation and enhancing metabolic function in cases of obesity.
POSTECH Professor Jong Kyoung Kim stated, “We have finally unraveled the mechanism governing TM4SF19 protein’s mechanism to regulate lysosomal activity.” He expressed optimism by saying, “Our discoveries may open new avenues for treating obesity and related metabolic disorders.”
The research was conducted with support from the Hanwoomul-Phagi Basic research Program, Medical Research Center Program, the National Bio-Resource Project, the Program for Key Research Institutes for Universities, Basic Research Laboratory Program, and the Program for Building a Foundation for Academic Research in Science and Engineering of the National Research Foundation of Korea, Korea University Medicine, and U.S. National Institutes of Health.
Journal
Nature Communications
DOI
10.1038/s41467-024-47108-8
Article Title
TM4SF19-mediated control of lysosomal activity in macrophages contributes to obesity-induced inflammation and metabolic dysfunction
Article Publication Date
30-Mar-2024
What's Your Reaction?

































