Rice team demonstrates miniature brain stimulator in humans
HOUSTON – (April 12, 2024) – Rice University engineers have developed the smallest implantable brain stimulator demonstrated in a human patient. Thanks to pioneering magnetoelectric power transfer technology, the pea-sized device developed in the Rice lab of Jacob Robinson in collaboration with Motif Neurotech and clinicians Dr. Sameer Sheth and Dr. Sunil Sheth can be powered wirelessly via an external […]
HOUSTON – (April 12, 2024) – Rice University engineers have developed the smallest implantable brain stimulator demonstrated in a human patient. Thanks to pioneering magnetoelectric power transfer technology, the pea-sized device developed in the Rice lab of Jacob Robinson in collaboration with Motif Neurotech and clinicians Dr. Sameer Sheth and Dr. Sunil Sheth can be powered wirelessly via an external transmitter and used to stimulate the brain through the dura ⎯ the protective membrane attached to the bottom of the skull.

Credit: Photo by Jeff Fitlow/Rice University
HOUSTON – (April 12, 2024) – Rice University engineers have developed the smallest implantable brain stimulator demonstrated in a human patient. Thanks to pioneering magnetoelectric power transfer technology, the pea-sized device developed in the Rice lab of Jacob Robinson in collaboration with Motif Neurotech and clinicians Dr. Sameer Sheth and Dr. Sunil Sheth can be powered wirelessly via an external transmitter and used to stimulate the brain through the dura ⎯ the protective membrane attached to the bottom of the skull.
The device, known as the Digitally programmable Over-brain Therapeutic (DOT), could revolutionize treatment for drug-resistant depression and other psychiatric or neurological disorders by providing a therapeutic alternative that offers greater patient autonomy and accessibility than current neurostimulation-based therapies and is less invasive than other brain-computer interfaces (BCIs).
“In this paper we show that our device, the size of a pea, can activate the motor cortex, which results in the patient moving their hand,” said Robinson, a professor of electrical and computer engineering and of bioengineering at Rice. “In the future, we can place the implant above other parts of the brain, like the prefrontal cortex, where we expect to improve executive functioning in people with depression or other disorders.”
Existing implantable technologies for brain stimulation are powered by relatively large batteries that need to be placed under the skin elsewhere in the body and connected to the stimulating device via long wires. Such design limitations require more surgery and subject the individual to a greater burden of hardware implantation, risks of wire breakage or failure and the need for future battery replacement surgeries.
“We eliminated the need for a battery by wirelessly powering the device using an external transmitter,” explained Joshua Woods, an electrical engineering graduate student in the Robinson lab and lead author on the study published in Science Advances. Amanda Singer, a former graduate student in Rice’s applied physics program who is now at Motif Neurotech, is also a lead author.
The technology relies on a material that converts magnetic fields into electrical pulses. This conversion process is very efficient at small scales and has good misalignment tolerance, meaning it does not require complex or minute maneuvering to activate and control. The device has a width of 9 millimeters and can deliver 14.5 volts of stimulation.
“Our implant gets all of its energy through this magnetoelectric effect,” said Robinson, who is founder and CEO of Motif, a startup working to bring the device to market. “The physics of that power transfer makes this much more efficient than any other wireless power transfer technologies under these conditions.”
Motif is one of several neurotech companies that are probing the potential of BCIs to revolutionize treatments for neurological disorders.
“Neurostimulation is key to enabling therapies in the mental health space where drug side effects and a lack of efficacy leave many people without adequate treatment options,” Robinson said.
The researchers tested the device temporarily in a human patient, using it to stimulate the motor cortex ⎯ the part of the brain responsible for movement ⎯ and generating a hand movement response. They next showed the device interfaces with the brain stably for a 30-day duration in pigs.
“This has not been done before because the quality and strength of the signal needed to stimulate the brain through the dura were previously impossible with wireless power transfer for implants this small,” Woods said.
Robinson envisions the technology being used from the comfort of one’s home. A physician would prescribe the treatment and provide guidelines for using the device, but patients would retain complete control over how the treatment is administered.
“Back home, the patient would put on their hat or wearable to power and communicate with the implant, push ‘go’ on their iPhone or their smartwatch and then the electrical stimulation from that implant would activate a neuronal network inside the brain,” Robinson said.
Implantation would require a minimally invasive 30-minute procedure that would place the device in the bone over the brain. Both the implant and the incision would be virtually invisible, and the patient would go home the same day.
“When you think about a pacemaker, it’s a very routine part of cardiac care,” said Sheth, professor and vice-chair of research, McNair Scholar and Cullen Foundation Endowed Chair of Neurosurgery at the Baylor College of Medicine. “In neurological and psychiatric disorders, the equivalent is deep brain stimulation (DBS), which sounds scary and invasive. DBS is actually quite a safe procedure, but it’s still brain surgery, and its perceived risk will place a very low ceiling on the number of people who are willing to accept it and may benefit from it. Here’s where technologies like this come in. A 30-minute minor procedure that is little more than skin surgery, done in an outpatient surgery center, is much more likely to be tolerated than DBS. So if we can show that it is about as effective as more invasive alternatives, this therapy will likely make a much larger impact on mental health.”
For some conditions, epilepsy for example, the device may need to be on permanently or most of the time, but for disorders such as depression and OCD, a regimen of just a few minutes of stimulation per day could suffice to bring about the desired changes in the functioning of the targeted neuronal network.
In terms of next steps, Robinson said that on the research side he is “really interested in the idea of creating networks of implants and creating implants that can stimulate and record, so that they can provide adaptive personalized therapies based on your own brain signatures.” From the therapeutic development standpoint, Motif Neurotech is in the process of seeking FDA approval for a long-term clinical trial in humans. Patients and caregivers can sign up on the Motif Neurotech website to learn when and where these trials will begin.
The work was supported in part by The Robert and Janice McNair Foundation, the McNair Medical Institute, DARPA and the National Science Foundation.
-30-
This news release can be found online at news.rice.edu.
Follow Rice News and Media Relations via Twitter @RiceUNews.
Peer-reviewed paper:
Miniature battery-free epidural cortical stimulators | Science Advances | DOI: 10.1126/sciadv.adn0858
Authors: Joshua Woods, Amanda Singer, Fatima Alrashdan, Wendy Tan, Chufeng Tan, Sunil Sheth, Sameer Sheth and Jacob Robinson
https://doi.org/10.1126/sciadv.adn0858
Video is available at:
https://youtu.be/jhAEpAJGKSE
(Video by Brandon Martin/Rice University)
Image downloads:
https://news-network.rice.edu/news/files/2024/04/240213_Implant_Fitlow_152-5b1ea18ebb9fdfc8.jpg
CAPTION: Rice University’s Jacob Robinson and his team of researchers have developed the smallest implantable brain stimulator demonstrated in a human patient that could revolutionize treatment for drug-resistant depression and other psychiatric or neurological disorders. (Photo by Jeff Fitlow/Rice University)
https://news-network.rice.edu/news/files/2024/04/JWF_8628-39b4feadc43c1c91.jpg
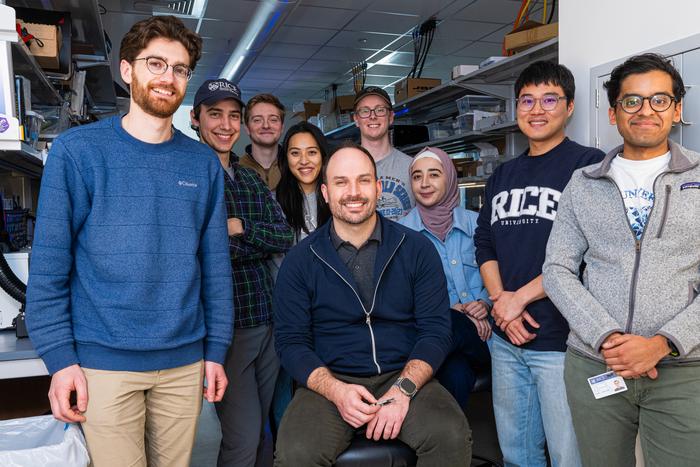
CAPTION: Joshua Woods (from left), Jacob Robinson and Fatima Alrashdan (Photo by Jeff Fitlow/Rice University)
https://news-network.rice.edu/news/files/2024/04/JWF_8453-a3f45ee916b1ce90.jpg
CAPTION: Rice University engineers have developed the first miniaturized brain stimulator shown to work in a human patient. (Photo by Jeff Fitlow/Rice University)
Box Link:
https://rice.box.com/s/il8b8lxzkekxxodzb1peqpjwcpv97a3c
Links:
Robinson lab: www.robinsonlab.com
Sheth lab: https://www.bcm.edu/research/faculty-labs/functional-and-cognitive-neurophysiology-laboratory
Motif Neurotech: www.motifneuro.tech
Rice Neuroengineering Initiative: neuroengineering.rice.edu
Rice Department of Electrical and Computer Engineering: eceweb.rice.edu
Rice Department of Bioengineering: https://bioengineering.rice.edu/
George R. Brown School of Engineering: engineering.rice.edu
About Rice:
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of architecture, business, continuing studies, engineering, humanities, music, natural sciences and social sciences and is home to the Baker Institute for Public Policy. With 4,574 undergraduates and 3,982 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction, No. 2 for best-run colleges and No. 12 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.
Journal
Science Advances
DOI
10.1126/sciadv.adn0858
Method of Research
Experimental study
Article Title
Miniature battery-free epidural cortical stimulators
Article Publication Date
12-Apr-2024
COI Statement
J.T.R., A.L.S., Sunil A. Sheth, Sameer A. Sheth, and J.E.W. receive monetary and/or equity compensation from Motif Neurotech. Sameer A. Sheth has consulting agreements with Zimmer Biomet, Boston Scientific, Koh Young, Neuropace, Varian, and Sensoria Therapeutics. Sunil A. Sheth has consulting agreements with Viz.AI, Penumbra, and Imperative Care as well as grant funding from Viz.AI and NIH. The terms of these arrangements have been reviewed and approved by Rice University, UTHealth, and Baylor college of Medicine in accordance with their policies on conflict of interest in research. J.T.R., F.A., J.E.W., and A.L.S. are coinventors on a provisional patent application filed by William Marsh Rice University (no. 18336787, filed 16 June 2023). The other authors declare that they have no competing interests.
What's Your Reaction?