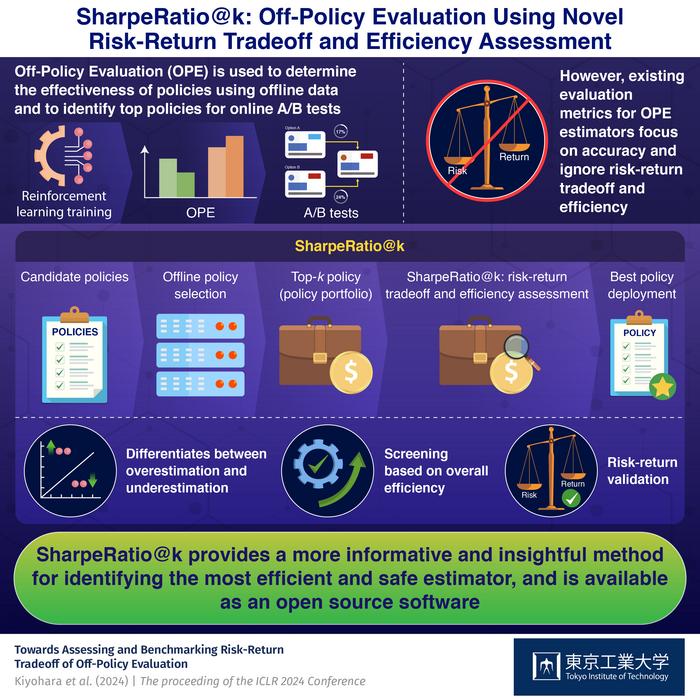
Obesity Drugs Aid Weight Loss After Bariatric Surgery
In recent years, bariatric surgery has emerged as one of the most effective interventions for severe obesity, offering sustained weight loss and significant improvements in obesity-related comorbidities. Despite its success, an increasingly recognized challenge in the postoperative management of bariatric patients is the phenomenon of recurrent weight gain or suboptimal clinical responses, which threaten the […]


In recent years, bariatric surgery has emerged as one of the most effective interventions for severe obesity, offering sustained weight loss and significant improvements in obesity-related comorbidities. Despite its success, an increasingly recognized challenge in the postoperative management of bariatric patients is the phenomenon of recurrent weight gain or suboptimal clinical responses, which threaten the long-term benefits of surgical intervention. This emerging concern has propelled a wave of clinical inquiry into the potential role of pharmacotherapy as a complementary tool to enhance and maintain weight loss outcomes after surgery. In a groundbreaking meta-analysis published in the International Journal of Obesity, researchers Golzarand, Toolabi, and Mirmiran provide a comprehensive synthesis of available evidence, striving to offer clinicians a practical guide to obesity medication regimens targeted at this vulnerable patient population.
Historically, bariatric surgery, which includes procedures such as Roux-en-Y gastric bypass, sleeve gastrectomy, and adjustable gastric banding, has been hailed for its profound effects on weight reduction and metabolic health. However, a subset of patients experiences weight regain several months or years following surgery. This can be attributed to a complex interplay of physiological, behavioral, and psychological factors, including anatomical changes, hormonal adaptations, and lifestyle elements that challenge the durability of initial weight loss. Persistent weight gain not only diminishes the quality of life but also exposes patients to renewed risk of cardiovascular disease, type 2 diabetes, and other obesity-related complications.
The meta-analysis conducted by Golzarand and colleagues represents a pivotal effort to collate data from multiple randomized controlled trials and observational studies, aiming to assess the efficacy and safety of obesity medications in patients who have undergone bariatric surgery but exhibit inadequate weight control thereafter. Notably, the authors highlight that despite the widespread use of pharmacotherapy in the general obese population, post-bariatric pharmacologic management remains controversial and lacks a consensus-driven protocol. This ambiguity stems in part from the heterogeneity of studied drug classes, variations in surgical techniques, and diverse patient characteristics.
One of the key insights from this extensive review is the therapeutic potential of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) in mitigating weight regain after surgery. These agents, originally developed for glycemic control in type 2 diabetes, have demonstrated marked anorectic effects, improved satiety, and enhanced energy expenditure. The meta-analysis underscores that administration of GLP-1 RAs postoperatively correlates with clinically meaningful reductions in body weight, a finding that may revolutionize postoperative obesity care by integrating pharmacologic adjuncts with surgical treatment.
In addition to GLP-1 receptor agonists, the comprehensive evaluation touches upon other pharmacological agents, including orlistat, phentermine-topiramate combination, and naltrexone-bupropion. Each of these drugs operates via distinct mechanisms: orlistat acts by inhibiting gastrointestinal lipases to reduce fat absorption; phentermine-topiramate influences central appetite regulation; and naltrexone-bupropion modulates reward pathways associated with eating behavior. The meta-analysis reveals variable but generally positive effects of these medications when used in the delicate postoperative phase, suggesting that personalized medication plans may be essential to optimize outcomes.
The research further delves into considerations surrounding timing, dosage, and duration of pharmacotherapy initiation after bariatric surgery. The lack of standardized guidelines has led to disparities in clinical practice, with some practitioners hesitant to prescribe obesity medications fearing adverse events or drug-surgery interactions. Golzarand et al. rigorously assess safety profiles, noting that the studied pharmacological agents exhibit acceptable tolerability, with side effects largely manageable and transient. Importantly, they emphasize a multidisciplinary approach involving surgeons, endocrinologists, dietitians, and mental health professionals to navigate the complexities of combined surgical and pharmacological obesity management.
Another salient point highlighted is the psychological dimension underpinning suboptimal postoperative weight loss. The meta-analysis calls attention to the role of pharmacotherapy in addressing not only physiological drivers of weight regain but also neurobehavioral and emotional factors that may compromise adherence to lifestyle modifications. In this context, medications affecting central nervous system pathways provide dual benefits: direct weight reduction and modulation of appetite-related cognitive processes.
The authors also discuss gaps in current research, advocating for more robust and large-scale clinical trials designed specifically to evaluate long-term outcomes of obesity medications post-bariatric surgery. Existing studies often suffer from heterogeneity in patient selection, follow-up duration, and outcome measures, limiting the extrapolation of findings to real-world clinical settings. Prospective, randomized trials with stratified patient cohorts are necessary to establish evidence-based protocols tailored to different surgical procedures and patient profiles.
In framing the meta-analysis as a practical guideline, Golzarand and colleagues propose an algorithmic approach for clinicians confronting postoperative weight regain. They recommend initiating pharmacotherapy when patients demonstrate significant weight regain — generally exceeding 10% of nadir weight — or when weight loss plateaus earlier than expected. This pragmatic framework underscores the importance of early intervention to prevent the re-emergence of obesity-related comorbidities and to sustain patient motivation by demonstrating continued support.
Crucially, the study also explores the cost-effectiveness implications of pharmacotherapy as an adjunct to bariatric surgery. While surgical procedures carry a substantial upfront cost, recurrent weight gain leads to increased healthcare utilization over time. By curtailing weight regain pharmacologically, the authors suggest potential downstream savings and enhanced long-term health economics, an argument likely to resonate with policymakers and insurance providers aiming to optimize resource allocation.
The meta-analysis does not ignore the pivotal role of patient education and sustained behavioral counseling. The authors advocate for integrating pharmacotherapy within a comprehensive obesity management program that prioritizes nutritional monitoring, physical activity encouragement, and psychological support. This holistic paradigm acknowledges that medication alone is insufficient unless embedded within a supportive care environment.
Broader implications of this research extend toward redefining post-bariatric clinical care standards globally. As obesity prevalence continues to rise and bariatric surgery remains a cornerstone treatment, standardized protocols to combat postoperative weight regain are urgently needed. The findings of this meta-analysis inspire optimism that combining surgical and pharmacological strategies can improve durability of weight loss, enhance patient quality of life, and reduce long-term health risks.
Moreover, the study opens avenues for future exploration of novel pharmacologic agents with enhanced efficacy and tailored safety profiles optimized for the postoperative context. Advances in precision medicine and an improved understanding of obesity pathophysiology at the molecular level may soon enable clinicians to individualize drug choice based on genetic, metabolic, and behavioral patient factors.
In conclusion, the meta-analysis by Golzarand, Toolabi, and Mirmiran represents a seminal contribution to obesity medicine, offering a nuanced synthesis of current evidence on the role of obesity medications following bariatric surgery. By bridging the gap between surgical success and long-term weight maintenance, this research provides an invaluable resource to clinicians seeking to confront the persistent challenge of postoperative weight regain. It heralds a new era in obesity treatment—one in which surgery and medication work synergistically to achieve sustainable health outcomes in this high-risk population.
Subject of Research:
Pharmacotherapy for managing recurrent weight gain and suboptimal clinical response in patients after bariatric surgery.
Article Title:
Obesity medications in patients with recurrent weight gain or suboptimal clinical response following bariatric surgery: a meta-analysis.
Article References:
Golzarand, M., Toolabi, K. & Mirmiran, P. Obesity medications in patients with recurrent weight gain or suboptimal clinical response following bariatric surgery: a meta-analysis.
Int J Obes (2025). https://doi.org/10.1038/s41366-025-01807-4
Image Credits: AI Generated
DOI: https://doi.org/10.1038/s41366-025-01807-4
Tags: bariatric surgery weight losslifestyle changes after bariatric surgerylong-term weight maintenance strategiesmeta-analysis on obesity drugsobesity medications after surgeryObesity-related comorbiditiespharmacotherapy for obesity managementpsychological factors in weight regainrecurrent weight gain post-surgeryRoux-en-Y gastric bypass outcomessleeve gastrectomy effectivenesssurgical intervention for severe obesity
What's Your Reaction?