Drug-like inhibitor shows promise in preventing flu
LA JOLLA, CA—Currently available flu medications only target the virus after it has already established an infection, but what if a drug could prevent infection in the first place? Now, scientists at Scripps Research and the Albert Einstein College of Medicine have designed drug-like molecules to do just that, by thwarting the first stage of […]
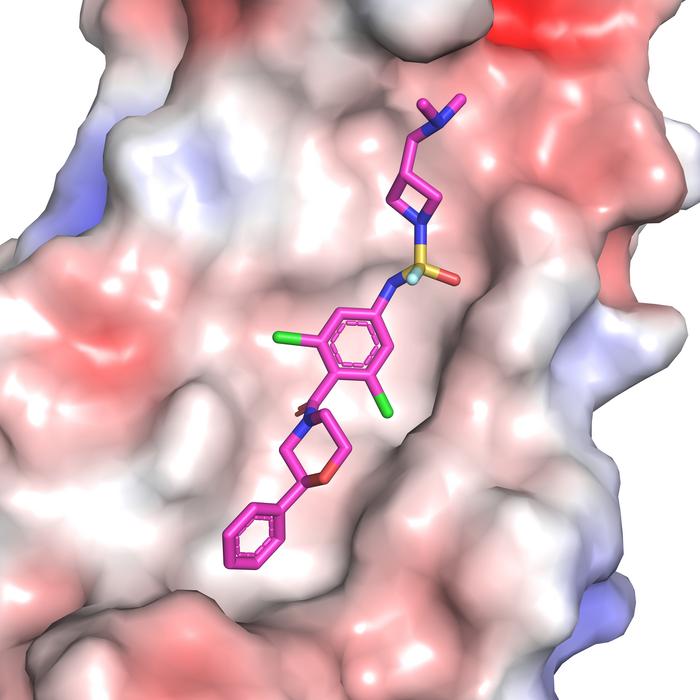
LA JOLLA, CA—Currently available flu medications only target the virus after it has already established an infection, but what if a drug could prevent infection in the first place? Now, scientists at Scripps Research and the Albert Einstein College of Medicine have designed drug-like molecules to do just that, by thwarting the first stage of influenza infection.

Credit: Scripps Research
LA JOLLA, CA—Currently available flu medications only target the virus after it has already established an infection, but what if a drug could prevent infection in the first place? Now, scientists at Scripps Research and the Albert Einstein College of Medicine have designed drug-like molecules to do just that, by thwarting the first stage of influenza infection.
The drug-like inhibitors block the virus from entering the body’s respiratory cells—specifically, they target hemagglutinin, a protein on the surface of type A influenza viruses. The findings, published in the Proceedings of the National Academy of Sciences on May 16, 2024, represent an important step forward in developing a drug that can prevent influenza infection.
“We’re trying to target the very first stage of influenza infection since it would be better to prevent infection in the first place, but these molecules could also be used to inhibit the spread of the virus after one’s infected,” says corresponding author Ian Wilson, DPhil, the Hansen Professor of Structural Biology at Scripps Research.
The inhibitors will need to be further optimized and tested before they can be assessed as antivirals in humans, but the researchers say that these molecules ultimately have the potential to help prevent and treat seasonal flu infections. And, unlike vaccines, the inhibitors likely wouldn’t need to be updated yearly.
The researchers had previously identified a small molecule, F0045(S), with a limited capacity to bind and inhibit H1N1 type A influenza viruses.
“We began by developing a high-throughput hemagglutinin binding assay that allowed us to rapidly screen large libraries of small molecules and found the lead compound F0045(S) with this process,” says corresponding author Dennis Wolan, PhD, senior principal scientist at Genentech and former associate professor at Scripps Research.
In this study, the team aimed to optimize F0045(S)’s chemical structure to design molecules with better drug-like properties and more specific binding ability to the virus. To start, the Wolan lab used “SuFEx click-chemistry,” which was first developed by two-time Nobel laureate and co-author K. Barry Sharpless, PhD, to generate a large library of candidate molecules with various tweaks to F0045(S)’s original structure. When they screened this library, the researchers identified two molecules—4(R) and 6(R)—with superior binding affinity compared to F0045(S).
Next, Wilson’s lab produced X-ray crystal structures of 4(R) and 6(R) bound to the flu hemagglutinin protein so that they could identify the molecules’ binding sites, determine the mechanisms behind their superior binding ability, and identify areas for improvement.
“We showed that these inhibitors bind much more tightly to the viral antigen hemagglutinin than the original lead molecule did,” says Wilson. “By using click-chemistry, we basically extended the compounds’ ability to interact with influenza by making them target additional pockets on the antigen surface.”
When the researchers tested 4(R) and 6(R) in cell culture to verify their antiviral properties and safety, they found 6(R) was non-toxic and had more than 200-times improved cellular antiviral potency compared to F0045(S).
Finally, the researchers used a targeted approach to further optimize 6(R) and develop compound 7, which proved to have even better antiviral ability.
“This is the most potent small-molecule hemagglutinin inhibitor developed to date,” says corresponding author Seiya Kitamura, who worked on the project as a postdoctoral fellow at Scripps Research and is now an assistant professor at the Albert Einstein College of Medicine.
In future studies, the team plans to continue to optimize compound 7 and to test the inhibitor in animal models of influenza.
“In terms of potency, it will be hard to improve the molecule any further, but there are many other properties to consider and optimize, for example, pharmacokinetics, metabolism and aqueous solubility,” says Kitamura.
Because the inhibitors developed in this study only target H1N1 strains of influenza, researchers are also working to develop equivalent drug-like inhibitors to target other strains of influenza such as H3N2 and H5N1.
In addition to Wilson, Wolan, Sharpless, and Kitamura, authors of the study, “Ultrapotent influenza hemagglutinin fusion inhibitors developed through SuFEx-enabled high-throughput medicinal chemistry” include Ting-Hui Lin, Chang-Chun David Lee, Akihiro Takamura, Rameshwar Kadam, Ding Zhang, Xueyong Zhu, Wenli Yu, and Yao Yao of Scripps Research; and Lucas Dada, Emiko Nagai of Albert Einstein College of Medicine
This work was supported by the NIH, the Nathan Shock Institute of Aging Research, and Einstein-Montefiore.
About Scripps Research
Scripps Research is an independent, nonprofit biomedical institute ranked one of the most influential in the world for its impact on innovation by Nature Index. We are advancing human health through profound discoveries that address pressing medical concerns around the globe. Our drug discovery and development division, Calibr-Skaggs, works hand-in-hand with scientists across disciplines to bring new medicines to patients as quickly and efficiently as possible, while teams at Scripps Research Translational Institute harness genomics, digital medicine and cutting-edge informatics to understand individual health and render more effective healthcare. Scripps Research also trains the next generation of leading scientists at our Skaggs Graduate School, consistently named among the top 10 US programs for chemistry and biological sciences. Learn more at www.scripps.edu.
Journal
Proceedings of the National Academy of Sciences
DOI
10.1073/pnas.2310677121
Article Title
Ultrapotent influenza hemagglutinin fusion inhibitors developed through SuFEx-enabled high-throughput medicinal chemistry
What's Your Reaction?

































