Valneva seeks Health Canada approval for chikungunya vaccine
The single-shot vaccine is intended for persons aged 18 years and above.
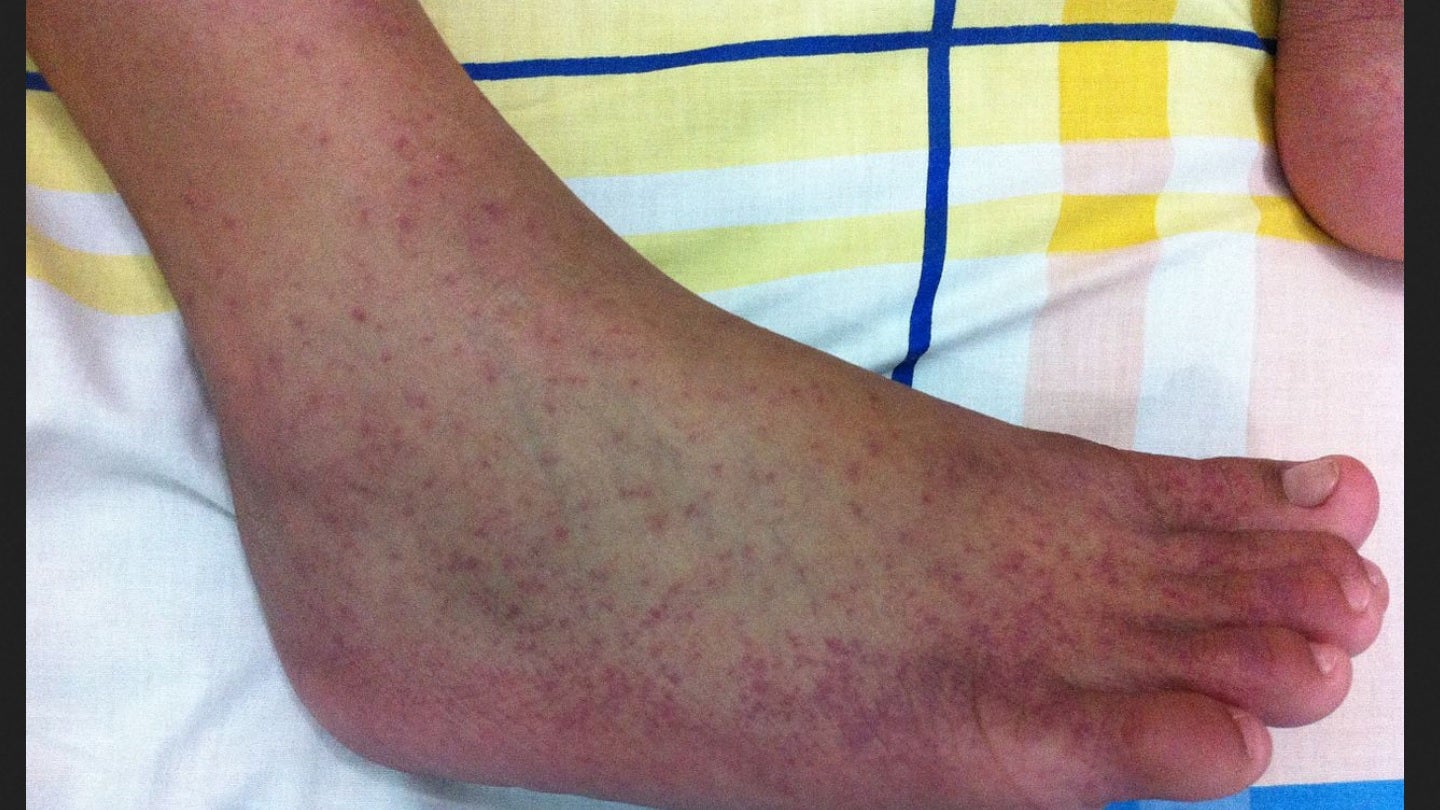
Valneva has submitted a regulatory application to Health Canada, seeking marketing approval for its chikungunya vaccine candidate, VLA1553.
The vaccine is designed to be administered as a single shot and is intended for individuals aged 18 years and older.
The latest application marks the company’s second regulatory application for VLA1553. Valneva plans to make further regulatory submissions.
The US Food and Drug Administration (FDA) is currently reviewing a biologic licence application (BLA) for the vaccine candidate under priority review.
It is expected to take action on the application by the end of August 2023.
Valneva chief medical officer Dr Juan Carlos Jaramillo stated: “Chikungunya represents a major threat for people travelling to or living in areas where chikungunya virus and the mosquitos that transmit it are present, including popular destinations for US and Canadian travellers.
“This threat continues to grow, as shown by the recent epidemiological alert of the Pan American Health Organisation (PAHO).”
The regulatory filings have been submitted to Health Canada and the FDA after the final pivotal Phase III data in March 2022, the final lot-to-lot consistency results in May 2022 and positive 12-month persistence data in December 2022.
In February 2023, Valneva completed enrolment and vaccination for a clinical study of VLA1553 in adolescents in Brazil. This trial is currently ongoing, with results expected by summer 2023.
The FDA awarded fast track designation for VLA1553 in 2018, and breakthrough therapy designation in 2021.
The vaccine candidate also received a priority medicine (PRIME) designation from the European Medicines Agency in 2020.
What's Your Reaction?