Understanding a broken heart
The stress of heart failure is remembered by the body and appears to lead to recurrent failure, along with other related health issues, according to new research. Researchers have found that heart failure leaves a “stress memory” in the form of changes to the DNA modification of hematopoietic stem cells, which are involved in the […]
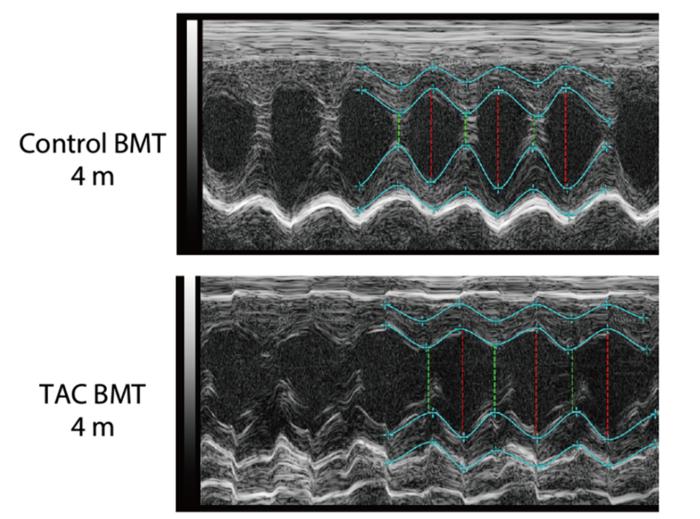
The stress of heart failure is remembered by the body and appears to lead to recurrent failure, along with other related health issues, according to new research. Researchers have found that heart failure leaves a “stress memory” in the form of changes to the DNA modification of hematopoietic stem cells, which are involved in the production of blood and immune cells called macrophages. These immune cells play an important role in protecting heart health. However, a key signaling pathway (a chain of molecules which relays signals inside a cell), called transforming growth factor beta (TGF-β), in the hematopoietic stem cells was suppressed during heart failure, negatively affecting macrophage production. Improving TGF-β levels could be a new avenue for treating recurrent heart failure, while detecting accumulating stress memory could provide an early warning system before it occurs.

Credit: 2024 Y. Nakayama, K. Fujiu, T. Oshima et al./ Science Immunology
The stress of heart failure is remembered by the body and appears to lead to recurrent failure, along with other related health issues, according to new research. Researchers have found that heart failure leaves a “stress memory” in the form of changes to the DNA modification of hematopoietic stem cells, which are involved in the production of blood and immune cells called macrophages. These immune cells play an important role in protecting heart health. However, a key signaling pathway (a chain of molecules which relays signals inside a cell), called transforming growth factor beta (TGF-β), in the hematopoietic stem cells was suppressed during heart failure, negatively affecting macrophage production. Improving TGF-β levels could be a new avenue for treating recurrent heart failure, while detecting accumulating stress memory could provide an early warning system before it occurs.
Healthier lives and improved well-being are among the United Nations’ global Sustainable Development Goals. Positively, a recent study shows that life expectancy worldwide is projected to increase by about 4.5 years by 2050. Much of this is thanks to public health efforts to prevent disease and improved survival from illnesses, such as cardiovascular disorders. However, heart disease is still the leading cause of death worldwide, with 26 million people estimated to be affected by heart failure.
Once heart failure has occurred, it has a tendency to reoccur along with other health issues, such as kidney and muscle problems. Researchers in Japan wanted to understand what causes this recurrence and the deterioration of other organs, and whether it can be prevented.
“Based on our earlier research, we hypothesized that recurrence may be caused by stress experienced during heart failure accumulating in the body, particularly in hematopoietic stem cells,” explained Project Professor Katsuhito Fujiu from the Graduate School of Medicine at the University of Tokyo. Hematopoietic stem cells are found in bone marrow and are the source of blood cells and a type of immune cell called macrophages, which help to protect heart health.
By studying mice with heart failure, the researchers found evidence of stress imprinting on the epigenome, that is, chemical changes occurred to the mice’s DNA. An important signaling pathway, called the transforming growth factor beta, which is involved in regulating many cellular processes, was suppressed in the hematopoietic stem cells of mice with heart failure, leading to the production of dysfunctional immune cells.
This change persisted over an extended period of time, so when the team transplanted bone marrow from mice with heart failure into healthy mice, they found that the stem cells continued to produce dysfunctional immune cells. The latter mice later developed heart failure and became prone to organ damage.
“We termed this phenomenon stress memory because the stress from heart failure is remembered for an extended period and continues to affect the entire body. Although various other types of stress might also imprint this stress memory, we believe that the stress induced by heart failure is particularly significant,” said Fujiu.
The good news is that by identifying and understanding these changes to the TGF-β signaling pathway, new avenues are now open for potential future treatments. “Completely new therapies could be considered to prevent the accumulation of this stress memory during hospitalization for heart failure,” said Fujiu. “In animals with heart failure, supplementing additional active TGF-β has been shown to be a potential treatment. Correcting the epigenome of hematopoietic stem cells could also be a way to deplete stress memory.”
Now that it has been identified, the team hopes to develop a system that can detect and prevent the accumulation of stress memory in humans, with a long-term goal of being able to not only prevent the recurrence of heart failure, but also catch the condition before it can fully develop. DOI: 10.1126/sciimmunol.ade3814
#####
Paper Title
Yukiteru Nakayama, Katsuhito Fujiu, Tsukasa Oshima, Jun Matsuda, Junichi Sugita, Takumi James Matsubara, Yuxiang Liu, Kohsaku Goto, Kunihiro Kani1, Ryoko Uchida, Norifumi Takeda, Hiroyuki Morita, Yingda Xiao, Michiko Hayashi, Yujin Maru, Eriko Hasumi, Toshiya Kojima, Soh Ishiguro, Yusuke Kijima, Nozomu Yachie, Satoshi Yamazaki, Ryo Yamamoto, Fujimi Kudo, Mio Nakanishi, Atsushi Iwama, Ryoji Fujiki, Atsushi Kaneda, Osamu Ohara, Ryozo Nagai, Ichiro Manabe, Issei Komuro. Heart failure promotes multimorbidity through innate immune memory. Science Immunology. 24 May 2024.
Useful Links:
Graduate School of Medicine: https://www.m.u-tokyo.ac.jp/english/
The University of Tokyo Hospital: https://www.h.u-tokyo.ac.jp/english/
Funding:
This study was supported by the Grant-in-Aid for Scientific Research from JSPS, AMED, and JST (JP 18K08061, 21K08122, 21ek0210157h0001 to Y.N.; JP17H05594, JP17H04171, 17H04171, 19K22615, 20ek0109423h0001, 22gm6510010h0002, 23ek0210193h0001 to K.F.; JP19H03648, JP20H04938, JP20K21594, JP21gm5010002 to I.M.; JPMJMS2023 to K.F. and I.M.; and JP20gm0810013, JP20ek0109440, JP20ek0109487, JP20ek0109406, JP20km0405209, JP20ek021014, JP20ek0210118, JP20ek0109367, JP19bm0804010 to I.K.), and grants from the MSD Life Science Foundation International, Japan Foundation for Applied Enzymology, the Japan Cardiovascular Research Foundation (to Y. N.), and the Takeda Science Foundation and Uehara Memorial Foundation (to. I.M.).
Competing interests
None.
Research Contact:
Project Professor Katsuhito Fujiu
Department of Advanced Cardiology
Graduate School of Medicine
The University of Tokyo, 7-3-1 Hongo
Bunkyo-ku, Tokyo, 113-8655, Japan
Tel.: +81-3-3815-5411
Email: fujiu-tky@g.ecc.u-tokyo.ac.jp
Press contact:
Mrs. Nicola Burghall (she/her)
Public Relations Group, The University of Tokyo,
7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8654, Japan
press-releases.adm@gs.mail.u-tokyo.ac.jp
About the University of Tokyo
The University of Tokyo is Japan’s leading university and one of the world’s top research universities. The vast research output of some 6,000 researchers is published in the world’s top journals across the arts and sciences. Our vibrant student body of around 15,000 undergraduate and 15,000 graduate students includes over 4,000 international students. Find out more at www.u-tokyo.ac.jp/en/ or follow us on X at @UTokyo_News_en.
Journal
Science Immunology
DOI
10.1126/sciimmunol.ade3814
Method of Research
Randomized controlled/clinical trial
Subject of Research
Animals
Article Title
Heart failure promotes multimorbidity through innate immune memory
Article Publication Date
24-May-2024
What's Your Reaction?

































