The future of drug testing: Vascularized organ-on-a-chip technologies
In an era marked by rapid technological advancement in biomedical engineering, a groundbreaking development is set to revolutionize our approach to drug testing and disease modeling. Researchers from Shanghai University and the University of California Los Angeles have made significant strides in the field of in vitro vascularized organ-on-a-chip systems, offering a promising alternative to […]
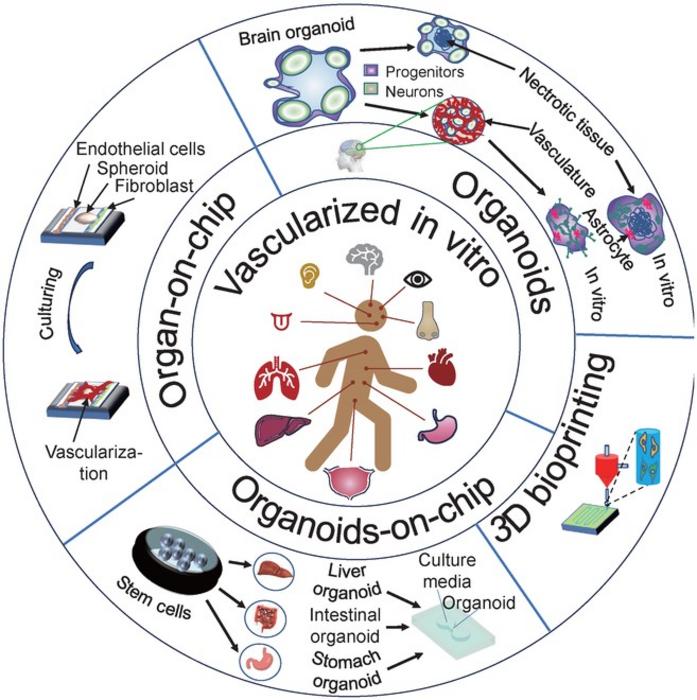
In an era marked by rapid technological advancement in biomedical engineering, a groundbreaking development is set to revolutionize our approach to drug testing and disease modeling. Researchers from Shanghai University and the University of California Los Angeles have made significant strides in the field of in vitro vascularized organ-on-a-chip systems, offering a promising alternative to traditional methods that rely heavily on animal testing and simplistic two-dimensional cell cultures.

Credit: Hongze Yin, School of Mechatronic Engineering and Automation, Shanghai University.
In an era marked by rapid technological advancement in biomedical engineering, a groundbreaking development is set to revolutionize our approach to drug testing and disease modeling. Researchers from Shanghai University and the University of California Los Angeles have made significant strides in the field of in vitro vascularized organ-on-a-chip systems, offering a promising alternative to traditional methods that rely heavily on animal testing and simplistic two-dimensional cell cultures.
The organ-on-a-chip technology mimics human organs on a microscale by cultivating cells in a controlled microenvironment that simulates the 3D structure and physiological functions of real tissues. These devices integrate various cell types within a network of tiny channels and chambers, allowing for precise manipulation of both the physical and chemical conditions.
The key to the effectiveness of these organ chips lies in their ability to incorporate vascular systems, which are essential for transporting nutrients, oxygen, and waste products—just like in the human body. This is achieved through sophisticated microengineering techniques such as 3D bioprinting, microfluidics, and the use of hydrogels that support the growth of microvascular networks. These networks are critical for creating more realistic organ models, as they enable the proper function and maturation of tissues, enhancing the chips’ biological relevance and their ability to mimic human responses accurately.
Recent innovations highlighted by the team include various types of organ chips such as those for the liver, brain, and heart, each designed to address specific research questions related to these organs. For instance, the liver chip allows for the study of drug metabolism and disease progression, while the brain chip offers insights into neurodegenerative diseases and the effects of pharmaceuticals on neural tissues.
One of the most significant advantages of vascularized organ-on-a-chip systems is their potential in personalized medicine. By using cells derived from individual patients, these chips can predict how specific patients will respond to different drugs, helping to tailor treatments to individual needs and minimize adverse effects. This could be particularly impactful in the treatment of complex diseases like cancer, where the efficacy and side effects of chemotherapy vary widely among patients.
Moreover, these devices hold the promise of reducing the reliance on animal testing, aligning with ethical considerations and potentially accelerating the drug development process. Traditional animal models often do not accurately replicate human disease states or predict human responses to treatments, leading to inefficiencies and high failure rates in drug development. Organ-on-a-chip technologies could provide a more accurate, ethical, and cost-effective solution.
The researchers also emphasize the role of these chips in studying multi-organ interactions and diseases involving several organ systems. By linking different organ chips through microfluidic channels, it’s possible to study the complex interactions between organs, offering deeper insights into systemic diseases and providing a holistic view of their progression and response to treatments.
In conclusion, vascularized organ-on-a-chip technologies represent a significant step forward in biomedical research, offering a powerful tool for drug testing, disease modeling, and personalized medicine. As these technologies continue to evolve, they promise to transform our approach to medical research, reducing our reliance on animal models, and providing more accurate, ethical, and efficient ways to develop new therapies for a wide range of diseases.
The paper, “Advances in the Model Structure of In Vitro Vascularized Organ-on-a-Chip,” was published in the journal Cyborg and Bionic Systems on Apr 25, 2024, at DOI: https://spj.science.org/doi/10.34133/cbsystems.0107.
Journal
Cyborg and Bionic Systems
DOI
10.34133/cbsystems.0107
Article Title
Advances in the Model Structure of In Vitro Vascularized Organ-on-a-Chip
Article Publication Date
25-Apr-2024
What's Your Reaction?

































