Structural engineering unlocks potent tumor treatment with dual-function magnetite nanozymes
According to a recent study published in Chemical Engineering Journal, a collaborative research team led by Professor WANG Hui from High Magnetic Field Laboratory, Hefei Institutes of Science of Chinese Academy of Sciences developed magnetite nanozyme (MNZs) with dual enzymatic activities through structural engineering, and proved its structure-dependent behavior in the process of tumor treatment. Credit: […]
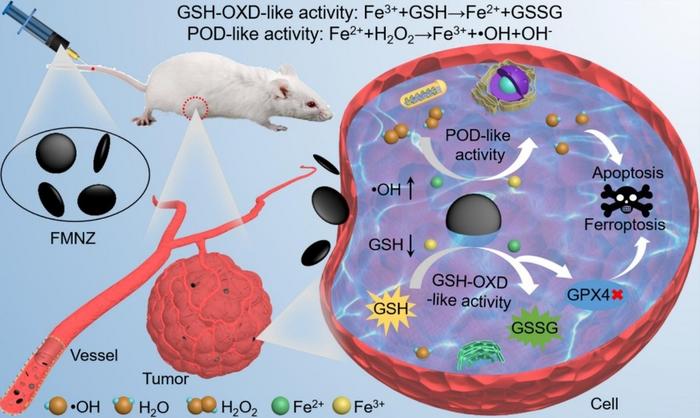
According to a recent study published in Chemical Engineering Journal, a collaborative research team led by Professor WANG Hui from High Magnetic Field Laboratory, Hefei Institutes of Science of Chinese Academy of Sciences developed magnetite nanozyme (MNZs) with dual enzymatic activities through structural engineering, and proved its structure-dependent behavior in the process of tumor treatment.

Credit: QIAN Yong
According to a recent study published in Chemical Engineering Journal, a collaborative research team led by Professor WANG Hui from High Magnetic Field Laboratory, Hefei Institutes of Science of Chinese Academy of Sciences developed magnetite nanozyme (MNZs) with dual enzymatic activities through structural engineering, and proved its structure-dependent behavior in the process of tumor treatment.
MNZs, as a substitute for natural enzymes, has been widely studied in the field of tumor catalytic therapy. However, the catalytic efficiency of traditional MNZs in tumor microenvironment (TME) is often limited, which is mainly due to the low production rate of hydroxyl radical (·OH).
In this study, the team developed MNZs with dual enzyme activity using solvothermal method.
“We prepared MNZs with three different shapes: flakes, ellipses and spheres,” said Prof. WANG hui, “and this allowed us to check out how the shapes affect the treatment and reveal the potential mechanism both in vitro and in vivo.”
The novel MNZs exhibit two important functions: They imitate glutathione, which is an antioxidant in cells. This helps to reduce the consumption of harmful hydroxyl radicals; and they act as peroxidases, breaking down hydrogen peroxide and generating highly toxic hydroxyl radicals. This self-cascade reaction disrupts the balance of reactive oxygen species in cells, enhancing the therapeutic effect on tumors.
This progress not only provides a new strategy for tumor catalytic therapy, but also opens up possibilities for the future application of nano-materials in the biomedical field, according to the team.
Journal
Chemical Engineering Journal
Article Title
Structural engineering of magnetite nanozymes for enhanced chemodynamic cancer therapy
Article Publication Date
7-May-2024
What's Your Reaction?