STAT+: Gilead’s CAR-T therapy prolongs survival in key blood cancer trial
Proving a meaningful survival benefit for patients with a type of blood cancer represents another milestone for CAR-T therapy. #ASCO23

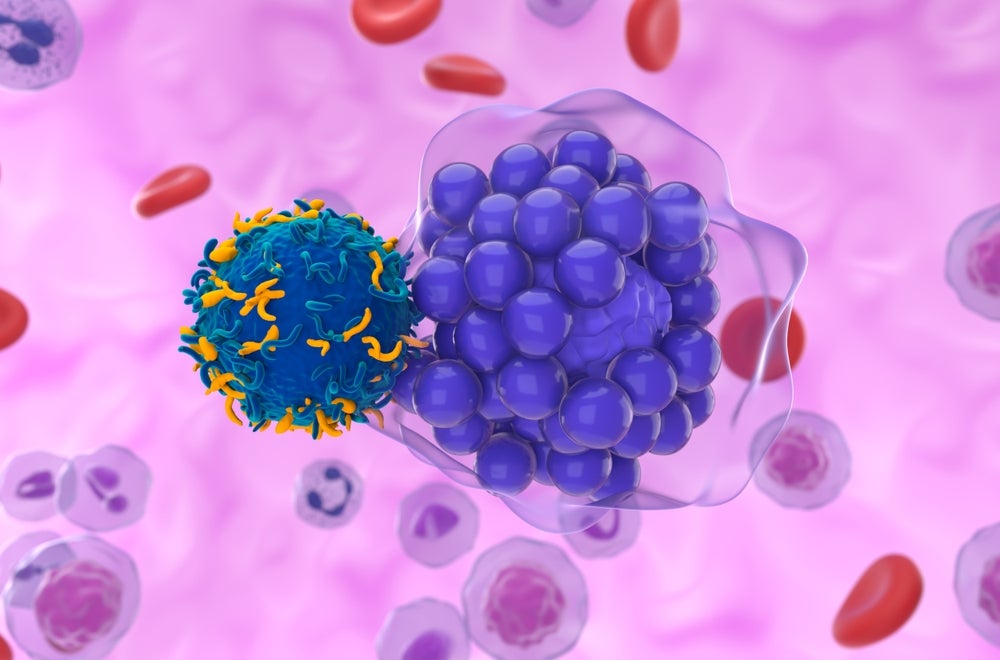
CHICAGO — Yescarta, the CAR-T therapy made by the Kite unit of Gilead Sciences, prolonged the lives of patients with large B-cell lymphoma by 27% compared to standard treatment in a long-running clinical trial, researchers reported Monday.
Proving a meaningful survival benefit for patients with a type of blood cancer represents another milestone for CAR-T therapy, which involves extracting white blood cells from a patient and genetically modifying them to attack cancer. Yescarta is the first CAR-T to do it in randomized study, which could help Gilead grow sales and extend its lead over competing cell therapies.
For nearly 30 years, chemotherapy and a stem cell transplant have been the standard treatments for people with large B-cell lymphoma that returns after initial, or first-line, treatment. But only about half of patients are eligible for this so-called second-line approach, and 20% are cured.
What's Your Reaction?