Soft X-ray absorption spectroscopy analysis of isolated water molecules within aqueous acetonitrile solutions
Herein, the O K-edge X-ray absorption spectroscopy (XAS) profile of an aqueous acetonitrile solution presented a distinct sharp peak not commonly observed in the corresponding profile of liquid water. Inner-shell calculations coupled with molecular dynamics simulations revealed that this sharp peak originated from isolated water molecules surrounded by acetonitrile molecules, rather than from water […]
Herein, the O K-edge X-ray absorption spectroscopy (XAS) profile of an aqueous acetonitrile solution presented a distinct sharp peak not commonly observed in the corresponding profile of liquid water. Inner-shell calculations coupled with molecular dynamics simulations revealed that this sharp peak originated from isolated water molecules surrounded by acetonitrile molecules, rather than from water clusters. Hence, O K-edge XAS could facilitate the electronic-structural analysis of isolated water molecules, differentiating their contributions from those of small water clusters.

Credit: Masanari Nagasaka
Herein, the O K-edge X-ray absorption spectroscopy (XAS) profile of an aqueous acetonitrile solution presented a distinct sharp peak not commonly observed in the corresponding profile of liquid water. Inner-shell calculations coupled with molecular dynamics simulations revealed that this sharp peak originated from isolated water molecules surrounded by acetonitrile molecules, rather than from water clusters. Hence, O K-edge XAS could facilitate the electronic-structural analysis of isolated water molecules, differentiating their contributions from those of small water clusters.
Water, the most abundant resource on Earth, exhibits unique properties owing to the presence of hydrogen bonds between its molecules. Specifically, the properties of water vary as it transitions from a liquid state to a cluster phase, influenced by different hydrogen-bonding structures. Hence, investigating the electronic structures of water clusters, particularly those of isolated water molecules, is crucial. In this direction, the current study subjected an aqueous acetonitrile (ACN) solution to O K-edge X-ray absorption spectroscopy (XAS) analysis, observing a sharp peak at approximately 537 eV in the resulting profile. Subsequently, based on the inner-shell calculations of water clusters derived from liquid structures obtained through molecular dynamics (MD) simulations of aqueous ACN solutions, the research team at Institute for Molecular Science aimed to determine if the observed sharp peak originated from completely isolated water molecules or small water clusters confined within ACN molecules.
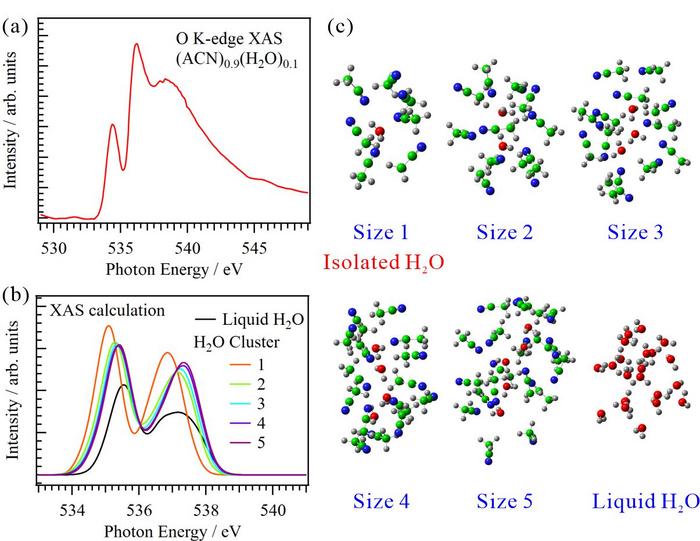
The XAS experiments were performed at the soft X-ray beamline BL3U of the UVSOR-III Synchrotron, utilizing a transmission-type liquid cell. Figure 1(a) illustrates the O K-edge XAS profile of an aqueous ACN solution [(ACN)0.9(H2O)0.1] recorded at 25°C. Notably, because ACN molecules lack oxygen atoms, the resulting O K-edge XAS profile is exclusively derived from water molecules. As depicted, the spectrum displays a sharp peak at approximately 537 eV, similar to the corresponding spectrum of water vapor. It is different form the spectrum of liquid water that features a broad peak at 537 eV owing to the presence of hydrogen bonds between water molecules separated by varying molecular distances.
Furthermore, Figure 1(b) depicts the O K-edge inner-shell spectra of liquid water and different-sized water clusters present in the aqueous ACN solution. Notably, the displayed O K-edge inner-shell spectrum of liquid water was obtained by summing 14,900 spectra of small water clusters, each comprising a soft-X-ray-excited central water molecule surrounded by other water molecules from the second coordination shell, as shown in Figure 1(c). Details regarding these structures were extracted from the MD simulations of liquid water conducted over 100 ns. Meanwhile, the inner-shell spectra of the different-sized water clusters, comprising isolated water molecules with a size of 1 and water clusters with sizes ranging from 2‒5, confined within ACN molecules were similarly obtained by summing 14,900 individual spectra of water clusters and ACN molecules in the first coordination shell (Figure 1(c)), extracting from the MD simulations of the aqueous ACN solution [(ACN)0.9(H2O)0.1] conducted over 100 ns. Interestingly, the inner-shell spectra of liquid water and water clusters with sizes ranging from 2‒5 exhibited similar spectral profiles, with the peak located at approximately 537 eV retaining a comparable energetic position. However, the inner-shell spectral profile of isolated water molecules differed from those of the aforementioned water clusters and liquid water. In particular, the peak located at 537 eV in the spectral profile of isolated water molecules shifted to a lower energy compared to that of liquid water. This suggests that the sharp peak observed in the O K-edge XAS profile of the aqueous ACN solution originated from isolated water molecules surrounded by ACN molecules, rather than from small water clusters.
While previous studies have already investigated the properties and dynamics of isolated water molecules, their methods have predominantly relied on complicated manipulations, involving encapsulating water molecules within fullerene or ionic liquids. In contrast, this study presents a relatively straightforward method, O K-edge XAS, for the electronic-structural analysis of isolated water molecules that are easily formed in aqueous ACN solutions.
Figure 1: (a) O K-edge XAS spectrum of an aqueous ACN solution [(ACN)0.9(H2O)0.1]. (b) Computed O K-edge inner-shell spectra of liquid water and different-sized water clusters present in the aqueous ACN solution. (c) Examples of the structures of the water clusters and liquid water are also illustrated. (Credit:Masanari Nagasaka, Restriction: CC BY)
Information of the paper:
Authors: Masanari Nagasaka
Journal Name: The Journal of Physical Chemistry Letters
Journal Title: “Probing Isolated Water Molecules in Aqueous Acetonitrile Solutions Using Oxygen K‑Edge X‑ray Absorption Spectroscopy”
DOI: https://doi.org/10.1021/acs.jpclett.4c01087
Financial Supports:
JSPS, KAKENHI, Grant-in-Aid for Scientific Research (B): JP19H02680
Joint Research by Institute for Molecular Science (IMS): 19-518
NINS, Okazaki Research Facilities, Research Center for Computational Science: 23-IMS-C199
Contact Person:
Masanari Nagasaka
Institute for Molecular Science, NINS
The Graduate Institute for Advanced Studies, SOKENDAI
TEL/FAX: +81-564-55-7394 / +81-564-55-7493
E-mail: nagasaka_at_ims.ac.jp (Please replace the “_at_” with @)
Journal
The Journal of Physical Chemistry Letters
DOI
10.1021/acs.jpclett.4c01087
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Probing Isolated Water Molecules in Aqueous Acetonitrile Solutions Using Oxygen K‑Edge X‑ray Absorption Spectroscopy
Article Publication Date
7-May-2024
What's Your Reaction?

































