Researchers identify key regulators underlying regeneration in Drosophila
Some animals possess the remarkable ability to regenerate lost structures, exemplified by a lizard regrowing its tail. However, this regenerative process must be tightly regulated by the body to ensure proper tissue organization and to prevent abnormal growths, such as cancer. Yet, the precise mechanisms underlying this regulation are not well known. In a recent […]
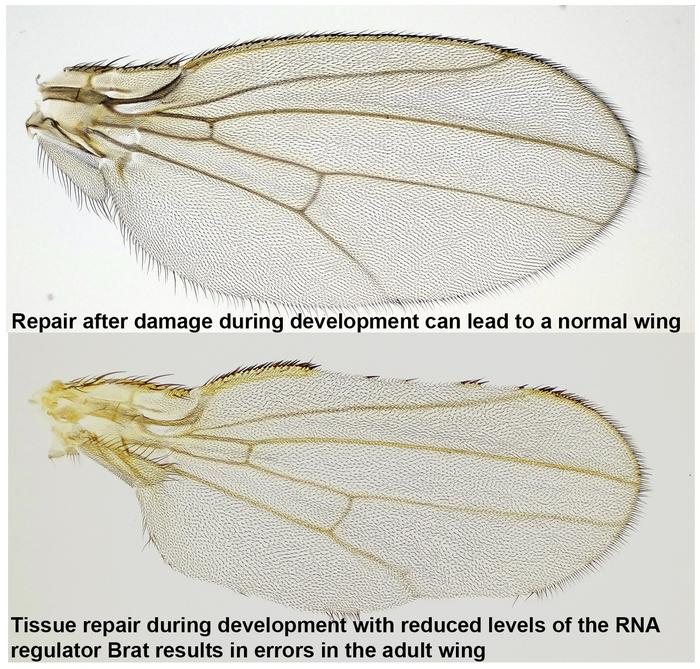
Some animals possess the remarkable ability to regenerate lost structures, exemplified by a lizard regrowing its tail. However, this regenerative process must be tightly regulated by the body to ensure proper tissue organization and to prevent abnormal growths, such as cancer. Yet, the precise mechanisms underlying this regulation are not well known. In a recent study published in PLOS Genetics, researchers at the University of Illinois Urbana-Champaign have identified an RNA-regulator called Brat as a key player in restraining tissue regeneration through its modulation of downstream growth factors.

Credit: The authors of the paper
Some animals possess the remarkable ability to regenerate lost structures, exemplified by a lizard regrowing its tail. However, this regenerative process must be tightly regulated by the body to ensure proper tissue organization and to prevent abnormal growths, such as cancer. Yet, the precise mechanisms underlying this regulation are not well known. In a recent study published in PLOS Genetics, researchers at the University of Illinois Urbana-Champaign have identified an RNA-regulator called Brat as a key player in restraining tissue regeneration through its modulation of downstream growth factors.
“There are constraints and protective factors that are important for making sure that regenerating tissue minimizes mistakes, but these haven’t been well studied,” said Rachel Smith-Bolton (GNDP/RBTE), an associate professor and associate head of cell and developmental biology. “When tissue regenerates, such as from a wound, even without any mutations, it sometimes makes mistakes, which I find really interesting. We want to explore what are the mistakes that can happen, and how can you protect against those mistakes.”
The team, led by Smith-Bolton, along with Syeda Nayab Fatima Abidi, a former graduate student in Smith-Bolton’s lab and first author on the study, and Felicity Ting-Yu Hsu, a current graduate student in the lab, investigated the genetic factors influencing regeneration of wing imaginal discs in Drosophila melanogaster, the common fruit fly. Drosophila larvae harbor imaginal discs, which serve as precursors for various appendages like wings, legs, and antennae. The intricate expression of genes within these discs dictates cell fate, or what appendage the cells will become, and the patterning.
Smith-Bolton says the process can be thought of in terms of growing a hand — the cells may be instructed to become fingers, but the patterning is what ensures you don’t end up with 5 thumbs rather than the usual fingers.
To determine the genes involved in this process, the researchers induced cell death in the wing imaginal discs of fly larvae, resulting in damaged wing discs that subsequently regenerated during development. By comparing wings of adult flies with various mutations to those of control flies, they pinpointed Brat, an mRNA regulator, as a crucial component in regenerative growth. Flies with a mutation that reduced Brat were better able to regenerate their developing wings compared to controls, indicating that Brat specifically works to restrain and control regenerative growth.
“The way fly genes are named is based on the mutant phenotype,” explained Abidi. “Brat gets its full name, Brain Tumor, because in mutants it causes tumors in the brain. This is because it controls whether stem cells are able to differentiate or not. However, there are no stem cells in wing imaginal discs, so it’s interesting that in our results Brat is still essentially performing the same kind of function, controlling whether and how much cells differentiate.”
While flies with reduced Brat demonstrated improved wing regeneration, this enhancement came with a trade-off: they exhibited a deficiency in bristles and veins within specific wing patches where damage had occurred. According to the researchers, this suggests a misstep in cell-fate specification at the wing margin, attributable to the unrestrained growth facilitated by reduced Brat expression.
Further investigation revealed that Myc, a downstream target of Brat and a growth factor, also plays a pivotal role in this process. Flies with Myc overexpression mirrored the phenotypes observed in Brat-reduced flies, underscoring the delicate balance required for proper regeneration.
“Brat reduces expression of its targets, and because Myc is a target of Brat, overexpressing Myc seems to result in the same phenotype as reducing Brat,” explained Smith-Bolton. “What was really interesting is no matter what we tried, we weren’t able to do the opposite and reduce Myc expression using our normal tools and tricks. This tells us that Myc is probably very tightly regulated in regenerating tissue.”
Hsu’s ongoing research focuses on elucidating Myc’s role in regeneration and its regulatory mechanisms. In her recent work, she was able to find an existing allele that causes underexpression of Myc in the flies. Surprisingly, this underexpression resulted in similar phenotypes to overexpression of Myc, suggesting a delicate balance in Myc’s expression is needed for proper regeneration.
“This just underscores the fact that you need the right amount of Myc during regeneration or you’re going to get mistakes,” said Smith-Bolton. “And we’re exploring now exactly what that amount is and how it’s regulated.”
Overall, the researchers concluded that Brat appears to act as a protective growth factor, constraining downstream growth factors such as Myc, and preventing errors in cell patterning and cell fate in regenerating tissue.
Given the presence of Brat orthologs — genes with similar function — in various species, including humans, these findings open the door for understanding and potentially manipulating regeneration in human contexts, particularly in curbing uncontrolled growth as seen in cancer.
“Though we didn’t look specifically at cancer, that is definitely the concern when you have a regenerative process that is unchecked, because the potential is that it could develop into a tumor,” said Abidi. “There have to be mechanisms in place that stop the process at the right time so that you are not just getting like a blob of growth, you’re getting something that’s functional. Uncovering the mutations that lead to unconstrained growth like this is a step towards understanding how those kinds of cancers develop.”
The study was funded by the National Institutes of Health and the Roy J. Carver Charitable Trust, can be found at https://doi.org/10.1371/journal.pgen.1011103
Journal
PLoS Genetics
DOI
10.1371/journal.pgen.1011103
Method of Research
Experimental study
Subject of Research
Animals
Article Title
Regenerative growth is constrained by brain tumor to ensure proper patterning in Drosophila
Article Publication Date
21-Dec-2023
What's Your Reaction?

































