Regenerative medicine: new focus on VEGF target?
According to GlobalData’s Drugs database, early-stage therapies are focused on vascular endothelial growth factor (VEGF) as the top drug target, with three drugs currently in development.
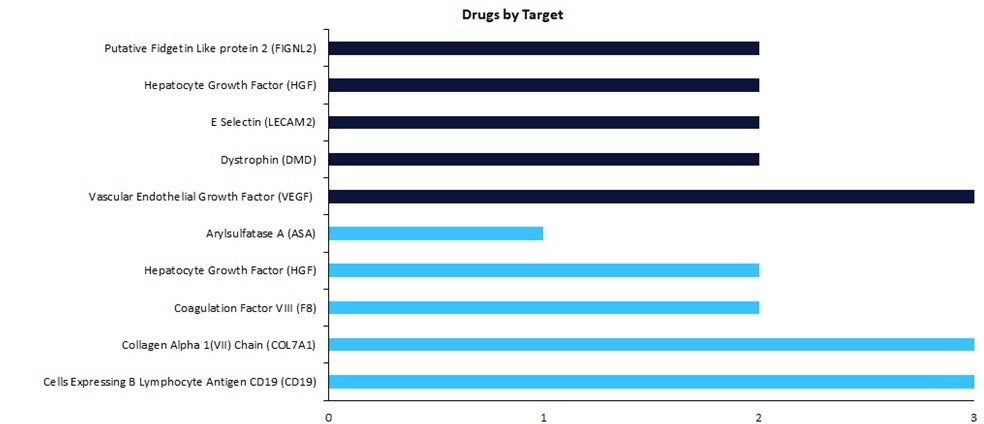
Regenerative medicines in early-stage development (preclinical, discovery, or investigational new drug [IND]/ clinical trial application [CTA] filed status) have seen a change in drug targets compared to therapies in late-stage development (Phase II to pre-registration stage). According to GlobalData’s Drugs database, early-stage therapies are focused on vascular endothelial growth factor (VEGF) as the top drug target, with three drugs currently in development.
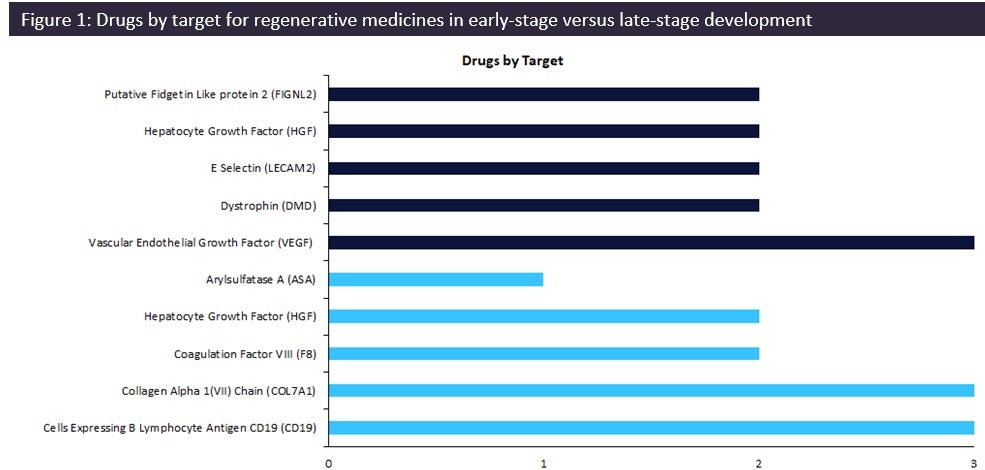
Note: Early stage is defined as therapies in preclinical, discovery, or IND/CTA filed status. Late-stage is defined as therapies in Phase II to pre-registration stage.
Dark blue: Key drug targets for therapies in early-stage development; light blue: key drug targets for therapies in late-stage development
Regenerative medicine is an approach of replacing or regenerating human cells, tissues, or organs to restore or establish normal function. Early-stage targets for regenerative medicine are currently led by VEGF due to its potential as a druggable target for multiple indications within both the central nervous system (CNS) and cardiovascular therapy areas. Surprisingly, the majority of the targets for drugs in early-stage development are not included in the top ten late-stage drug targets, highlighting a shift in the targets for regenerative medicines.
As demonstrated by Figure 1, collagen Alpha 1(VII) chain (COL7A1) and Cells Expressing B Lymphocyte Antigen CD19 lead the late-stage targets with three drugs in development each. Two key regenerative medicines in late stages are beremagene geperpavec, a gene therapy by Krystal Biotech for the treatment of epidermolysis bullosa, a rare dermatological condition, and Yescarta, a gene-modified cell therapy by Gilead Sciences indicated for nodal marginal zone B-cell lymphoma, B-cell non-Hodgkin lymphoma, and extranodal marginal zone B-cell lymphoma, with both therapies currently in pre-registration.
VEGF is an angiogenic protein with neurotrophic and neuroprotective actions, with the increased focus on VEGF as a regenerative medicine target attributed to its potential use as a target to help CNS indications such as Alzheimer’s disease (AD) and Parkinson’s disease, two common neurodegenerative diseases with severe unmet need, currently affecting more than 55 million and ten million people worldwide, respectively. One of the regenerative medicines in early-stage development focusing on VEGF as a target, ‘Encapsulated VEGF Secreting Cells’ by the University of the Basque Country, a gene-modified cell therapy, aims to elicit these angiogenic properties and improve cognitive impairment. Over the last 30 years, there have been only nine innovative drugs approved for the treatment of AD; only 11% were gene cell therapies. Staggeringly, none of the 61 drugs approved for Parkinson’s within the last 20 years were gene therapies.
There is a distinct shift in drug targets between early- and late-stage therapies, with drugs moving away from COL7A1 and CD19 to the VEGF target. However, gene therapies remain key molecule types for these targets. Thus, positive outcomes for these gene therapies including those in early-stage development targeting VEGF may instigate changes in the marketed landscape, resulting in diversified targets within the drug market across therapy areas such as oncology and CNS.
What's Your Reaction?