Novel theranostic tool allows for noninvasive identification and treatment of ovarian cancer
Reston, VA—A new radiotheranostic system has the ability to detect and treat ovarian cancer noninvasively, according to new research published in the April issue of The Journal of Nuclear Medicine. Combining the highly specific huAR9.6 antibody with PET and therapeutic radionuclides, this theranostic platform may provide more personalized treatment to improve health outcomes for ovarian cancer […]
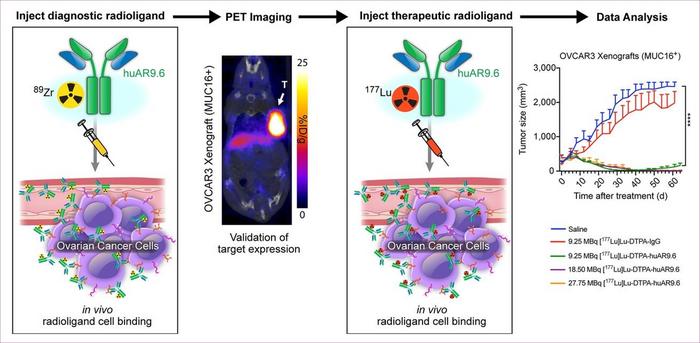
Reston, VA—A new radiotheranostic system has the ability to detect and treat ovarian cancer noninvasively, according to new research published in the April issue of The Journal of Nuclear Medicine. Combining the highly specific huAR9.6 antibody with PET and therapeutic radionuclides, this theranostic platform may provide more personalized treatment to improve health outcomes for ovarian cancer patients.

Credit: © 2023 Memorial Sloan-Kettering Cancer Center, Memorial Hospital for Cancer and Allied Diseases, and Sloan-Kettering Institute for Cancer Research, each in New York, NY. All rights reserved. Republished with permission.
Reston, VA—A new radiotheranostic system has the ability to detect and treat ovarian cancer noninvasively, according to new research published in the April issue of The Journal of Nuclear Medicine. Combining the highly specific huAR9.6 antibody with PET and therapeutic radionuclides, this theranostic platform may provide more personalized treatment to improve health outcomes for ovarian cancer patients.
Ovarian cancer causes more deaths than any other gynecologic malignancy, with a five-year survival rate below 30 percent for patients diagnosed at advanced stages. The current standard of care for ovarian cancer consists of surgery followed by platinum-based chemotherapy; however, these methods have failed to increase overall survival rates in patients because of tumor recurrence and chemoresistance.
“Current serum-based biomarkers do not sufficiently detect all occurrences of early-stage ovarian cancer,” stated Jason Lewis, PhD, Chief Attending of Radiochemistry and Emily Tow Chair at Memorial Sloan Kettering Cancer (MSK) in New York, New York. “Therefore, there is a critical need for both additional detection methods and new targeted therapies that can improve patient survival.”
Studies have shown that the MUC16 protein is overexpressed in ovarian cancer patients, with elevated levels correlating with disease stage and tumor volume. The antibody huAR9.6 binds to a unique epitope that is influenced by truncated carbohydrate residues on MUC16. Thus, the authors noted, MUC16 could be a potential target for tumor detection via immuno-PET imaging and treatment with radioimmunotherapy.
In the study, the diagnostic radiotracer 89Zr-DFO-huAR9.6 was investigated in vitro and in vivo via binding experiments, immuno-PET imaging, and biodistribution studies on ovarian cancer mouse models. In addition, ovarian xenografts were used to determine the safety and efficacy of the therapeutic radionuclide, 177Lu-CHX-A″-DTPA-huAR9.6.
MUC16 proteins were successfully detected via immuno-PET imaging with 89Zr-DFO-huAR9.6. In vivo studies showed that 89Zr-DFO-huAR9.6 could effectively specify varying levels of MUC16 expression in ovarian cancer mouse models. Radioimmunotherapy studies with 177Lu-CHX-A″-DTPA-huAR9.6 demonstrated improved overall survival and strong antitumor responses in highly MUC16-expressing models. Hematologic toxicity was also determined to be transient in mice treated with 177Lu-CHX-A″-DTPA-huAR9.6.
“Immuno-PET imaging of MUC16 with this radiotheranostic pair may allow for noninvasive diagnosis and treatment monitoring of ovarian cancer lesions in patients,” said Kyeara Mack, PhD, postdoctoral fellow in the Lewis Lab at MSK. “This theranostic platform may be used to stratify and select patients who would benefit from the targeted radioimmunotherapy. In addition, it could also play a significant role in early ovarian cancer detection.”
This study was made available online in March 2024.
The authors of “Interrogating the Theranostic Capacity of a MUC16-Targeted Antibody for Ovarian Cancer” include Kyeara N. Mack, Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, New York, and Department of Pharmacology, Weill Cornell Graduate School of Medical Sciences, Weill Cornell Medicine, New York, New York; Zachary V. Samuels, Lukas M. Carter, Tara D. Viray, and Komal Mandleywala, Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, New York; Cory L. Brooks, Department of Chemistry and Biochemistry, California State University, Fresno, California; Michael A. Hollingsworth and Prakash Radhakrishnan, Eppley Institute for Research in Cancer and Allied Diseases, University of Nebraska Medical Center, Omaha, Nebraska; and Jason S. Lewis, Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, New York, Department of Pharmacology, Weill Cornell Graduate School of Medical Sciences, Weill Cornell Medicine, New York, New York, and Molecular Pharmacology Program, Memorial Sloan Kettering Cancer Center, New York, New York.
Visit the JNM website for the latest research, and follow our new Twitter and Facebook pages @JournalofNucMed or follow us on LinkedIn.
###
Please visit the SNMMI Media Center for more information about molecular imaging and precision imaging. To schedule an interview with the researchers, please contact Rebecca Maxey at (703) 652-6772 or rmaxey@snmmi.org.
About JNM and the Society of Nuclear Medicine and Molecular Imaging
The Journal of Nuclear Medicine (JNM) is the world’s leading nuclear medicine, molecular imaging and theranostics journal, accessed more than 16 million times each year by practitioners around the globe, providing them with the information they need to advance this rapidly expanding field. Current and past issues of The Journal of Nuclear Medicine can be found online at http://jnm.snmjournals.org.
JNM is published by the Society of Nuclear Medicine and Molecular Imaging (SNMMI), an international scientific and medical organization dedicated to advancing nuclear medicine and molecular imaging—precision medicine that allows diagnosis and treatment to be tailored to individual patients in order to achieve the best possible outcomes. For more information, visit www.snmmi.org.
Journal
Journal of Nuclear Medicine
DOI
10.2967/jnumed.123.266524
Article Title
Interrogating the Theranostic Capacity of a MUC16-Targeted Antibody for Ovarian Cancer
Article Publication Date
1-Apr-2024
COI Statement
The humanization of AR9.6 was funded by a private research contract to Cory Brooks from Quest Pharmatech Inc. during the conduct of the study. Jason Lewis reports grants from the NIH (R35 CA232130), Emerson Collective Cancer Research Fund, Tow Foundation, and IMRAS Memorial Sloan Kettering Cancer Center during this study. This study was also supported in part by the NIH R35 CA232130-S2 Diversity Supplement, awarded to Kyeara Mack. The Radiochemistry and Molecular Imaging Probes Core Facility, the Small Animal Imaging Facility, and the Molecular Cytology Core Facility were supported in part by NIH P30 CA08748. Prakash Radhakrishnan and Michael Hollingsworth acknowledge the NIH for support (CA208108 and P01CA217798).
What's Your Reaction?