New tool provides researchers with improved understanding of stem cell aging in the brain
MADISON — Researchers can use the light naturally thrown off by biological specimens to better study the different states of stem cells in the nervous system, thanks to a tool developed at the University of Wisconsin–Madison, brightening their chances for studying the way stem cells age. Credit: Image courtesy of Darcie Moore MADISON — Researchers […]
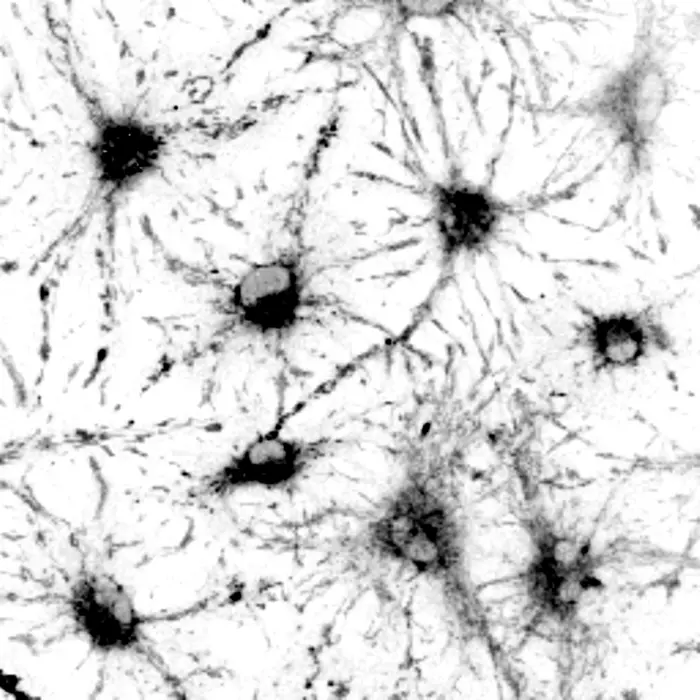
MADISON — Researchers can use the light naturally thrown off by biological specimens to better study the different states of stem cells in the nervous system, thanks to a tool developed at the University of Wisconsin–Madison, brightening their chances for studying the way stem cells age.
Credit: Image courtesy of Darcie Moore
MADISON — Researchers can use the light naturally thrown off by biological specimens to better study the different states of stem cells in the nervous system, thanks to a tool developed at the University of Wisconsin–Madison, brightening their chances for studying the way stem cells age.
The UW–Madison team combined autofluorescence — that natural light emission — and sequencing genetic material in single cells to study the behavior of neural stem cells. Autofluorescence is often considered a hindrance, as it can obscure the glowing labels researchers use to track specific signals within a cell. In their new technique, however, the researchers found the signatures of autofluorescence can be used to study stem cells’ dormant state, known as quiescence.
They published their findings recently in the journal Cell Stem Cell.
“The quiescent state is very important, because the exit from quiescence is the rate-limiting step in making newborn neurons in the adult brain. Aging and neurological diseases limit this exit from quiescence, so we have a great need to study adult neural stem cells in their different cell states,” says Darcie Moore, the senior author on the study, a professor of neuroscience and member of UW–Madison’s Stem Cell and Regenerative Medicine Center. “Our goal was to create a new tool that could identify if an adult neural stem cell was quiescent and its different substates (dormant or resting quiescence), or if the cell is activated, entering into the cell cycle.”
Moore partnered with Melissa Skala, a UW–Madison biomedical engineering professor, Morgridge Institute for Research investigator and member of the Stem Cell and Regenerative Medicine Center whose lab has been developing fluorescence lifetime imaging to study the autofluorescent signatures associated with single cells.
When cells shift from active to quiescent states, the presence and abundance of certain proteins important to metabolism change. These molecules alter the way light is absorbed and emitted back out of the cell. By focusing on light emitted by parts of the cell that change in key ways with quiescence, the researchers identified the light “signature” that matches a target cell state.
“These natural signals within the cell can reliably identify cell function and identity,” Skala says. “It’s like nature is trying to tell us all the secrets of life.”
By sequencing RNA — a kind of working copy of DNA used to produce the proteins that make things happen in cells — in the mouse neural stem cells they studied, the researchers confirmed matches between cell state and light signatures.
By identifying and decoding these autofluorescence signatures, Moore and Skala have developed a tool that can aid in studying adult neurological diseases and aging, but potentially also expand beyond neuroscience. They’ve already begun working with Colin Crist, a professor of human genetics at McGill University, to investigate the unique autofluorescent signatures present in muscle stem cells.
“Now that we’ve discovered that this research created not only a tool but gave us unique insight to cellular processes that are different between quiescent and activated neural stem cells, I feel even more strongly that identifying a cell based on how they act versus how they express one protein will shift studies from studying static systems to dynamic systems,” says Moore. “That we can study these cells as they change throughout time without destroying them — while also seeing how these functional measures change — is very exciting.”
This research was supported in part by grants from the National Institutes of Health (P30 CA014520, 1S10RR025483-01, T32GM008688 and 1DP2OD025783) as well as the Vallee Foundation, Morgridge Institute for Research and Retina Research Foundation.
###
Journal
Cell Stem Cell
Method of Research
Experimental study
Subject of Research
Cells
What's Your Reaction?