Microparticle analysis: methods and uses in the pharmaceutical industry
Particle analysis on a micro-scale is a significant topic in several fields of application. Microparticles can be present as a…

Particle analysis on a micro-scale is a significant topic in several fields of application. Microparticles can be present as a contamination of products such as pharmaceuticals and electronics, or as an air pollutant. Alternatively, they can come as a product component of formulations such as cleaning agents and polymers.
Within the pharmaceutical industry, it is critical to measure particle size as it influences surface area and porosity, impacting a drug’s bioavailability, effectiveness, and shelf life. Particle size is monitored in quality control as well as the development of active pharmaceutical ingredients (APIs). Some applications, such as powder inhalers for the treatment of lung diseases, require particle analysis as a critical aspect of their development.
IR microscopy for easy analysis of fibres, particles and residues
There are several methods used for particle analysis, with each one having its own specialities and being suited to different applications. Among the most prominent methods are infrared (IR) imaging and single-point measurements.
IR imaging involves analysing complete filters on which particles are prepared in their entirety, using spectral data from samples to determine their chemical properties. Most molecules absorb light in the IR region of the electromagnetic spectrum, converting it to molecular vibration, and this absorption characterises the nature of the chemical bonds contained in a sample. In IR imaging, a spectrometer is used to measure the absorption as a function of wavelength; this creates an IR spectrum that can act as a characteristic ‘molecular fingerprint’ to identify organic and inorganic samples. This method is particularly well-suited to processing high amounts of particles, such as in environmental samples.
One advantage offered by IR microscopy is that it allows fibres, particles and residues to be identified easily. For example, many automobiles are painted using special paint that features many different layers, which have very specific tasks to give the vehicle a unique appearance. This means that if a car is targeted in a hit-and-run incident, the microscopic paint chips can be analysed to identify the perpetrator by tracking their vehicle.
Methods and purposes of single-point spectroscopy
Single-point spectroscopic methods include Raman spectroscopy, Fourier-Transform infrared (FT-IR) microscopy and IR laser spectroscopy. These involve acquiring one spectrum at each particle’s positions, which requires the particles to be visually localised beforehand based on contrast. They are generally better suited for lower particle loads, such as tap water.
For instances when particles need to be analysed individually or cannot be obtained in bulk, FT-IR microscopy can act as a powerful technique to determine the chemical identity of particles. FT-IR microscopy allows an IR spectrum to be measured from anywhere on the sample with a very high local resolution, meaning even complex multi-component particle compositions can be analysed. Even a single particle can be enough for chemical analysis, unlike with many other common analytical techniques, which require more sample material. In addition, FT-IR measurement is non-destructive and other analytical techniques can be applied afterwards if necessary.
One significant disadvantage of single-point micro-spectroscopy is that it requires particles to be visually recognisable before they can be analysed. Because of this, tiny and transparent particles can be easily overlooked, while particle agglomerates (which are not properly separated) are usually detected as one big particle instead.

Combination of IR laser imaging and FT-IR spectroscopy
IR laser imaging and FT-IR spectroscopy are increasingly used alongside each other to increase imaging accuracy and gather results more quickly. Although both methods provide characteristic IR information, IR laser imaging using quantum cascade lasers (QCLs) offers much greater power density than the thermal IR sources typically used in FT-IR spectroscopy. This enables faster imaging speeds by increasing device sensitivity. QCLs can, however, only provide analysis within a limited spectral range of 1,800-950cm-1, so they should be used in combination with FT-IR spectroscopy to ensure analytical confidence.
Using IR laser imaging in combination with FT-IR allows users to quickly locate regions of interest, measure them, and identify substances with no ambiguity. While IR imaging focuses on the MIR fingerprint region of 1,800-950cm-1, FT-IR microscopy can access a broader spectral range of 450-6,000cm-1; this allows unknown substances to be identified while improving reliability. The two techniques can be used in transmission, reflection and attenuated total reflection (ATR).
Instrumentation for FT-IR imaging and other particle analysis
Bruker Optics, a division of the US-based Bruker Corporation, offers several solutions for FT-IR imaging and other particle analysis methods. Among these is the LUMOS II, a fully motorised FT-IR microscope that features an integrated spectrometer. LUMOS II offers a high level of automation and makes use of state-of-the-art optics for optimal sample visualisation and infrared data collection; the microscope’s eight-fold objective provides the capacity for attenuated total reflectance ATR, transmission, reflection and high-quality visual inspection.
The LUMOS II II has proven an ideal tool for testing various substances for contamination in pharmaceutical contexts. One trial of the device involved filtering contamination out of a sample using a gold filter measuring around 5μm in pore size; after the contaminant had been dried, it was examined directly on the gold-coated substrate using the LUMOS II II. The image below shows the results of the sample analysis using the microscope, with the filter pores represented by small dots on the right of the image and the filtration residue appearing as black particles and fibres.
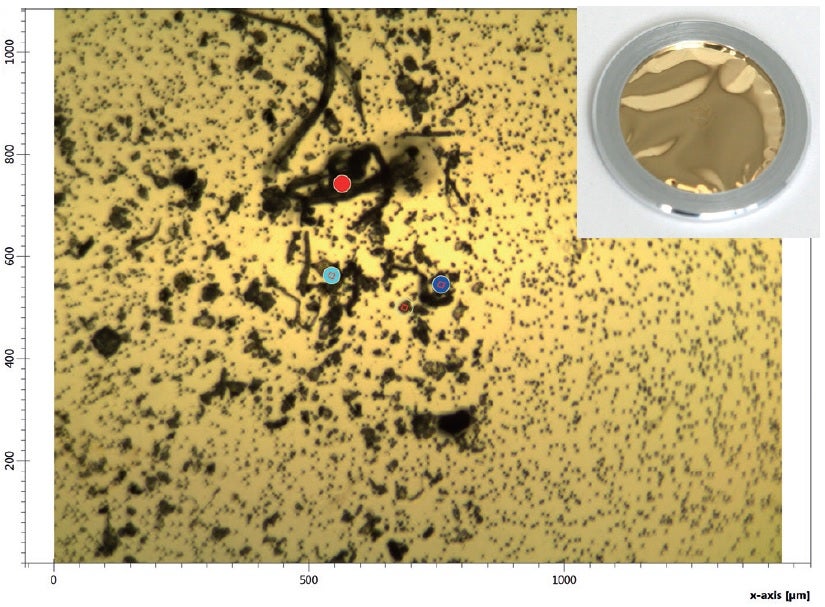
IR laser imaging with no limits to MIR information
Although IR laser imaging can deliver high-quality chemical images in very little time, it cannot provide all the MIR information that is sometimes needed to identify samples reliably. This is especially true for inorganic pigments and filler materials, which often show their main spectral characteristics only below 1,000cm-1.
Bruker Optics’ HYPERION II removes this limitation and enables both IR laser imaging and μ-FT-IR to be carried out using one system. The device uses a broadband MCT to perform microscopic IR analysis to a minimum of 450cm-1, which is often necessary in forensic sciences. In addition, the workflow is entirely integrated and allows users to switch between measurement modes in one click, as well as utilise both modes in parallel.
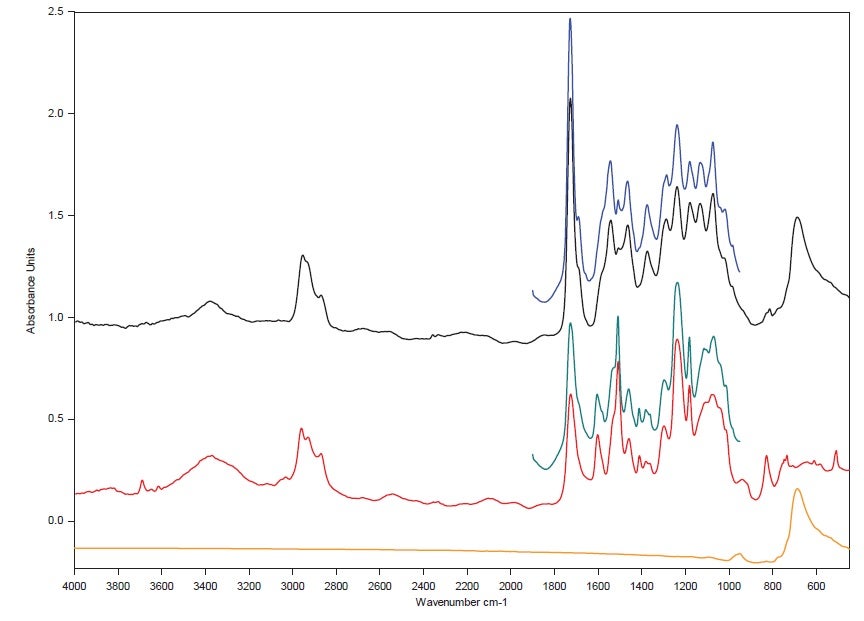
The graph below shows all the FT-IR spectra acquired through IR laser image creation with HYPERION II compared with the IR laser spectra ‒ the MIR region is in full accordance and the analysis took less than 15 minutes in total.
Choose Bruker for the best in particle size analysis instrumentation
IR imaging, single-point measurement and other methods of particle analysis are being used increasingly in the development of new drugs, in which the size of particles helps to determine a product’s flowability, content uniformity, absorption behaviour and other factors, as well as affecting tabletting and granulation processes. Bruker’s solutions are designed to meet all particle analysis needs by providing a wide range of features and capabilities – the LUMOS II microscope includes an integrated spectrometer and advanced optics to enhance sample visualisation and data collection, while the HYPERION II simplifies the particle analysis process by combining IR laser imaging and FT-IR in one device.
For more information about the LUMOS II, HYPERION II or any of our other FT-IR instruments, please contact us today by visiting our company profile (linked at the top of this page).
What's Your Reaction?

































