Manganese sprinkled with iridium: a quantum leap in green hydrogen production
As the world is transitioning from a fossil fuel-based energy economy, many are betting on hydrogen to become the dominant energy currency. But producing “green” hydrogen without using fossil fuels is not yet possible on the scale we need because it requires iridium, a metal that is extremely rare. In a study published May 10 in […]
As the world is transitioning from a fossil fuel-based energy economy, many are betting on hydrogen to become the dominant energy currency. But producing “green” hydrogen without using fossil fuels is not yet possible on the scale we need because it requires iridium, a metal that is extremely rare. In a study published May 10 in Science, researchers led by Ryuhei Nakamura at the RIKEN Center for Sustainable Resource Science (CSRS) in Japan report a new method that reduces the amount of iridium needed for the reaction by 95%, without altering the rate of hydrogen production. This breakthrough could revolutionize our ability to produce ecologically friendly hydrogen and help usher in a carbon-neutral hydrogen economy.

Credit: RIKEN
As the world is transitioning from a fossil fuel-based energy economy, many are betting on hydrogen to become the dominant energy currency. But producing “green” hydrogen without using fossil fuels is not yet possible on the scale we need because it requires iridium, a metal that is extremely rare. In a study published May 10 in Science, researchers led by Ryuhei Nakamura at the RIKEN Center for Sustainable Resource Science (CSRS) in Japan report a new method that reduces the amount of iridium needed for the reaction by 95%, without altering the rate of hydrogen production. This breakthrough could revolutionize our ability to produce ecologically friendly hydrogen and help usher in a carbon-neutral hydrogen economy.
With 70% of the world covered in water, hydrogen is truly a renewable source of energy. However, extracting hydrogen from water on a scale that can rival fossil fuel-based energy production is not yet possible. Current global energy production is almost 18 terawatts, meaning that at any given moment, about 18 trillion watts of power is being produced on average worldwide. For alternative green methods of energy production to replace fossil fuels, they must be able to reach the same rates of energy production.
The green way to extract hydrogen from water is an electrochemical reaction that requires a catalyst. The best catalysts for this reaction—the ones that yield the highest rate and the most stable hydrogen production—are rare metals, with iridium being the best of the best. But the scarcity of iridium is a big problem. “Iridium is so rare that that scaling up global hydrogen production to the terawatt scale is estimated to require 40 years’ worth of iridium,” says co-first author Shuang Kong.
The Biofunctional Catalyst Research Team at RIKEN CSRS is trying to get around the iridium bottleneck and find other ways of producing hydrogen at high rates for long periods of time. In the long run, they hope to develop new catalysts based on common earth metals, which will be highly sustainable. In fact, the team recently succeeded in stabilizing green hydrogen production at a relatively high level using a form of manganese oxide as a catalyst. However, achieving industrial level production in this manner is still years away.
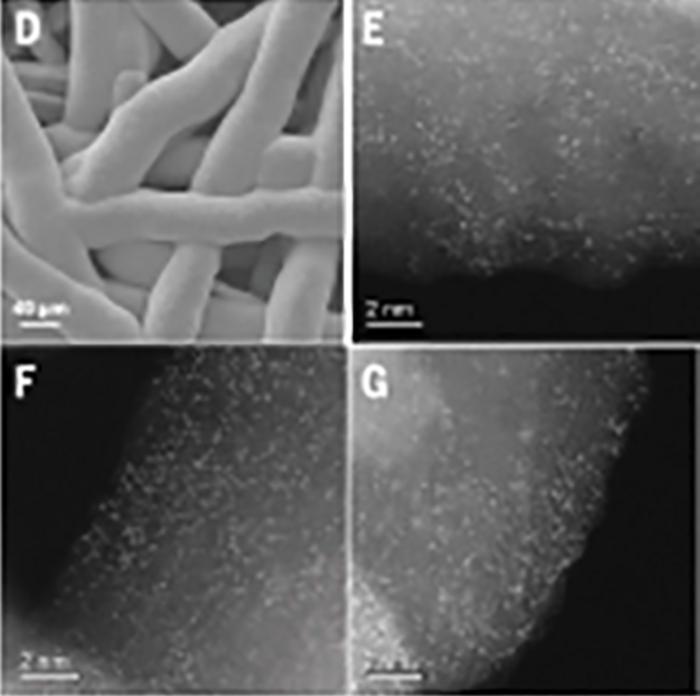
“We need a way to bridge the gap between rare metal- and common metal-based electrolyzers, so that we can make a gradual transition over many years to completely sustainable green hydrogen,” says Nakamura. The current study does just that by combining manganese with iridium. The researchers found that when they spread out individual iridium atoms on a piece of manganese oxide so that they didn’t touch or clump with each other, hydrogen production in a proton exchange membrane (PEM) electrolyzer was sustained at the same rate as when using iridium alone, but with 95% less iridium.
With the new catalyst, continuous hydrogen production was possible for over 3000 hours (about 4 months) at 82% efficiency without degradation. “The unexpected interaction between manganese oxide and iridium was key to our success,” says co-author Ailong Li. “This is because the iridium resulting from this interaction was in the rare and highly active +6 oxidation state.”
Nakamura believes that the level of hydrogen production achieved with the new catalyst has high potential for immediate usefulness. “We expect our catalyst to be easily transferred to real-world applications,” he says, “which will immediately increase the capacity of current PEM electrolyzers.”
The team has begun collaborating with partners in industry, who have already been able to improve on the initial iridium-manganese catalyst. Moving forward, the RIKEN CSRS researchers plan to continue investigating the specific chemical interaction between iridium and manganese oxide, with hopes of reducing the amount of necessary iridium even more. At the same time, they will continue collaborating with industrial partners, and plan on deploying and testing the new catalyst on an industrial scale in the near future.
Journal
Science
DOI
10.1126/science.adg5193
Article Title
Atomically dispersed hexavalent iridium oxide from MnO2 reduction for oxygen evolution catalysis
Article Publication Date
10-May-2024
What's Your Reaction?

































