Key protein regulates immune response to viruses in mammal cells
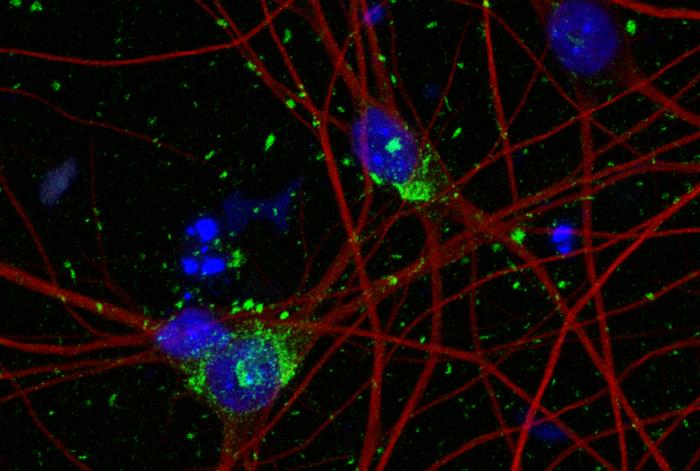
Researchers have revealed the regulatory mechanism of a specific protein that plays a key role in balancing the immune response triggered by viral infections in mammal cells. These findings could help drive the development of antiviral therapies and nucleic acid medicines to treat genetic disorders. Credit: Tomoko Takahashi (Saitama University/The University of Tokyo) and Kumiko […]
Researchers have revealed the regulatory mechanism of a specific protein that plays a key role in balancing the immune response triggered by viral infections in mammal cells. These findings could help drive the development of antiviral therapies and nucleic acid medicines to treat genetic disorders.

Credit: Tomoko Takahashi (Saitama University/The University of Tokyo) and Kumiko Ui-Tei (The University of Tokyo)
Researchers have revealed the regulatory mechanism of a specific protein that plays a key role in balancing the immune response triggered by viral infections in mammal cells. These findings could help drive the development of antiviral therapies and nucleic acid medicines to treat genetic disorders.
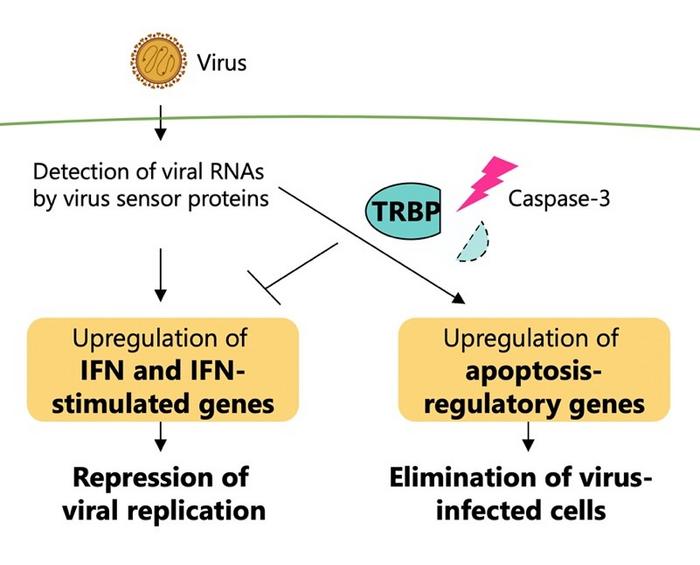
For cells to protect themselves from viral infections, a series of immune responses typically occur, including programmed cell death called apoptosis and interferon signaling. While apoptosis is a normal process, which occurs with or without the presence of viral molecules, following a cascade of steps to end with the death of a cell — which might not sound advantageous to the host — it can help prevent the reproduction of abnormal cells, including those infected by viruses, and eliminate them from the body. Interferons, on the other hand, are proteins produced by animal cells in response to a viral infection to protect the cell against viral attacks and prevent the virus from replicating. The regulatory mechanism of how cells maintain a balance between apoptosis and interferon response to efficiently suppress viral replication during infection, however, remained unclear.
In the current study, a team including researchers from the University of Tokyo focused on a specific protein, TRBP, which is also classified as a type of protein called an RNA silencing factor.
RNA is a nucleic acid, an organic compound found in living cells and viruses, which controls protein synthesis and the genetic makeup of many viruses. RNA synthesizes proteins through a process known as translation, by reading genetic sequences and translating them into instructions for the cells to create proteins, which are mostly responsible for the overall structure and function of the organism, whether it’s a plant or animal.
RNA silencing, also known as RNA interference, is a way that plants and invertebrate animals can protect themselves from viruses by cleaving viral RNA to repress viral replication.
“This study provides a significant insight that clearly revealed the protein related to the RNA silencing mechanism, which is known to be an antiviral mechanism in a plant or invertebrate, is strongly related to antiviral response also in mammals by another mechanism,” said co-author Tomoko Takahashi, a visiting researcher at the University of Tokyo and assistant professor at Saitama University in Japan.
Though it is widely understood that RNA silencing is a mechanism that operates under normal conditions to control gene expression (if the gene is “turned on” to provide instructions for the cell to assemble the specific protein it encodes), it’s still unclear how this process occurs under the stress of viral infection.
So the researchers looked at TRBP (an abbreviation for TAR RNA-binding protein), which has shown a significant role in RNA silencing during a viral infection.
This protein interacts with a virus sensor protein early on in the phases of infection in human cells. In the later stages of viral infection, proteins called caspases are activated, and this type of protein is chiefly responsible for triggering cell death.
“RNA silencing and interferon signaling were previously considered as independent pathways, but multiple reports, including ours, have demonstrated crosstalk between them,” said Kumiko Ui-Tei, another co-author and associate professor from the University of Tokyo (at the time of the study).
This functional conversion of TRBP triggered by viral infection is the basis of regulating interferon response and apoptosis, with TRBP irreversibly increasing the programmed cell death of infected cells, while reducing interferon signaling. TRBP works on the cell by inducing cell death, stopping the viral replication entirely, in contrast to the interferon response pathway, which just subdues viral replication instead of eliminating the infected cells.
“The ultimate goal is understanding the molecular mechanism underlying the antiviral defense system, orchestrated through the interplay between internal and external RNA pathways in human cells,” said Takahashi.
By gaining a deeper understanding of how defenses against viruses work on a molecular level, the researchers aim to drive the development of nucleic acid medicines. These medicines utilize targeting and inhibition approaches similar to the antiviral response of RNA silencing, and they hold promise of being increasingly useful in treating a wider range of patients afflicted with viral infections, genetic mutations and genetic defects.
This study was conducted in collaboration with Saitama University, Chiba University, Kyoto University and Maebashi Institute of Technology in Japan.
###
Journal article:
Keiko Shibata, Harune Moriizumi, Koji Onomoto, Yuka Kaneko, Takuya Miyakawa, Shuhei Zenno, Masaru Tanokura, Mitsutoshi Yoneyama, Tomoko Takahashi, Kumiko Ui-Tei, “Caspase-mediated processing of TRBP regulates apoptosis during viral infection,” Nucleic Acids Research: April 19, 2024, DOI: 10.1093/nar/gkae246
Link: https://doi.org/10.1093/nar/gkae246
Funding:
Ministry of Education, Culture, Sports, Science and Technology of Japan, SECOM Science and Technology Foundation, Takeda Science Foundation, MSD Life Science Foundation, Naito Science and Engineering Foundation, Canon Foundation, Japan Health and Research Institute, Hitachi Global Foundation, and Joint Usage/Research Program of Medical Mycology Research Center
Related links:
Graduate School of Science
Research contacts:
Tomoko Takahashi
Visiting Researcher, Graduate School of Science, The University of Tokyo
Assistant Professor, Saitama University
Email: takahas@mail.saitama-u.ac.jp
Kumiko Ui-Tei
Project Researcher, Graduate School of Science, The University of Tokyo
Email: ktei@bs.s.u-tokyo.ac.jp
Press contact:
Joseph Krisher
Public Relations Group, The University of Tokyo
Email: press-releases.adm@gs.mail.u-tokyo.ac.jp
About the University of Tokyo
The University of Tokyo is Japan’s leading university and one of the world’s top research universities. The vast research output of some 6,000 researchers is published in the world’s top journals across the arts and sciences. Our vibrant student body of around 15,000 undergraduate and 15,000 graduate students includes over 4,000 international students. Find out more at https://www.u-tokyo.ac.jp/en/ or follow us on X (formerly Twitter) at @UTokyo_News_en
Journal
Nucleic Acids Research
DOI
10.1093/nar/gkae246
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Caspase-mediated processing of TRBP regulates apoptosis during viral infection
Article Publication Date
19-Apr-2024
What's Your Reaction?