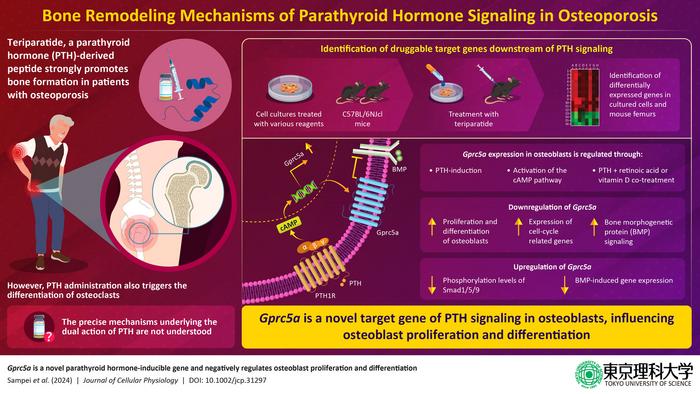
Innovative Diagnostic Tool Employs Bioluminescence to Identify Viruses
A Revolutionary Leap in Viral Diagnostics: The Emergence of LUCAS Bioluminescence Technology In a breakthrough that could redefine the future of point-of-care diagnostics, researchers at Mass General Brigham have unveiled an innovative technology known as the Luminescence CAscade-based Sensor, or LUCAS. This newly developed diagnostic tool harnesses the power of amplified bioluminescence to detect viral […]


A Revolutionary Leap in Viral Diagnostics: The Emergence of LUCAS Bioluminescence Technology
In a breakthrough that could redefine the future of point-of-care diagnostics, researchers at Mass General Brigham have unveiled an innovative technology known as the Luminescence CAscade-based Sensor, or LUCAS. This newly developed diagnostic tool harnesses the power of amplified bioluminescence to detect viral particles rapidly, accurately, and with unprecedented sensitivity within complex biological samples. Its development marks a critical advance in overcoming long-standing barriers intrinsic to traditional diagnostic assays, promising transformative impacts on viral detection and patient care worldwide.
The challenge in viral diagnostics has always been the extreme difficulty in identifying tiny infectious agents amidst the complexity of biological fluids such as blood or mucus. Dr. Hadi Shafiee, an engineering faculty member at Brigham and Women’s Hospital and a leading figure behind LUCAS, likens this difficulty to “finding an ice cube in a jelly-filled Olympic swimming pool while blindfolded.” This vivid analogy underscores the fundamental problem extrinsic to conventional viral assays: sensitivity and accuracy are often compromised by the minuscule concentration of viral particles and the intricate nature of the biological milieu.
.adsslot_vNAF7y9uZa{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_vNAF7y9uZa{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_vNAF7y9uZa{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
Traditional bioluminescence assays employ the luciferase enzyme—best known for the luminescent glow of fireflies—to illuminate biological samples, thereby flagging the presence of targeted molecules such as viral antigens. When luciferase interacts with its substrate luciferin, it generates a brief burst of light indicating a reaction. Despite the elegant simplicity of this natural mechanism, its practical application in diagnostics has been hindered by the inherently weak and transient nature of the emitted light signal. This significant limitation has curtailed its deployment in sensitive, point-of-care viral detection.
Addressing this bottleneck, the pioneering research team engineered a novel enzyme cascade strategy that dramatically intensifies and prolongs bioluminescent signals. By integrating beta-galactosidase, an enzyme that binds to luciferin and facilitates its continuous release, into the luciferase reaction system, LUCAS effectively creates a biochemical feedback loop. This cascade ensures that luciferin molecules are not squandered in one-off reactions but instead are steadily liberated to sustain multiple light-generating interactions. The result is a robust amplification of bioluminescence, making the signal approximately 500 times stronger and eight times longer lasting than prior assays.
The ramifications of this enhanced bioluminescence system are profound. In rigorous testing with an extensive array of viral-spiked patient and serum samples—totaling over 300 specimens infected with clinically significant pathogens such as SARS-CoV-2, HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV)—LUCAS demonstrated remarkable diagnostic performance. The assay delivered results swiftly, averaging under 23 minutes per test, while maintaining an impressive accuracy exceeding 94% across all pathogen types. This level of sensitivity and speed positions LUCAS as a potent tool especially beneficial for environments lacking sophisticated laboratory infrastructure.
Beyond technical prowess, LUCAS was deliberately designed with portability and user accessibility in mind. Its adaptability makes it suitable for deployment across diverse healthcare settings—from under-resourced clinics to technologically advanced hospitals. This versatility addresses a critical need in global health: providing reliable, rapid diagnostics at the point of care to facilitate timely clinical decision-making and curtail the spread of infectious diseases.
As infectious diseases evolve and new pathogens continue to emerge, diagnostic platforms must be both flexible and forward-compatible. The LUCAS platform’s modular enzyme cascade approach holds promising potential for multiplexed pathogen detection, allowing for simultaneous identification of multiple infectious agents within a single sample. Furthermore, researchers envision expanding its application beyond viruses to recognize biomarkers linked to a spectrum of diseases, including neurodegenerative conditions like Alzheimer’s disease, thereby broadening its clinical utility.
The significance of early detection in managing infectious diseases cannot be overstated. Prompt diagnosis enables timely therapeutic interventions that can dramatically improve patient outcomes and reduce transmission. By melding cutting-edge bioengineering with enzymology, LUCAS exemplifies the forefront of personalized medicine diagnostics, making early, sensitive, and accurate detection more accessible than ever.
Behind this innovation is a multidisciplinary team, including a cadre of talented scientists such as first author Dr. Sungwan Kim and collaborators spanning biomedical engineering, clinical medicine, and molecular diagnostics. Their concerted efforts culminated in a peer-reviewed publication detailing LUCAS’s capabilities in the prestigious journal Nature Biomedical Engineering, reflecting robust scientific validation and credibility.
Notably, while celebrating this advancement, ethical considerations accompany groundbreaking technologies. The inventors have filed a patent through Brigham and Women’s Hospital to protect the intellectual property embodied in LUCAS, a reflection of its proprietary nature and potential commercial impact.
Supported by significant funding from the National Institutes of Health, this research evidences how strategic investment in biomedical engineering can yield practical, lifesaving technologies. The convergence of expertise in enzyme kinetics, immunoassays, and biomedical instrumentation has fundamentally reshaped the landscape of viral diagnostics.
Looking ahead, the research community anticipates further development and clinical testing phases that will evaluate LUCAS’s performance in detecting viral pathogens in a broader range of bodily fluids and real-world patient populations. Its potential to revolutionize diagnostic protocols promises to contribute substantially to global efforts against current and future pandemics.
As we stand on the cusp of this diagnostic revolution, the advent of LUCAS affirms the transformative power of bioluminescent technologies and enzyme cascade engineering. Such innovations are vital in transcending the limits of existing methodologies, ultimately empowering clinicians and patients with rapid, accurate, and accessible viral detection tools that could save countless lives.
Subject of Research: Rapid, ultrasensitive bioluminescence immunoassay technology for point-of-care viral antigen detection.
Article Title: Ultrasensitive and long-lasting bioluminescence immunoassay for point-of-care viral antigen detection
News Publication Date: 30-May-2025
Web References:
– https://www.massgeneralbrigham.org/
– https://www.nature.com/articles/s41551-025-01405-9
References:
Kim S et al. “Ultrasensitive and long-lasting bioluminescence immunoassay for point-of-care viral antigen detection.” Nature Biomedical Engineering. DOI: 10.1038/s41551-025-01405-9
Keywords: Biomedical engineering, bioluminescence, point-of-care diagnostics, viral detection, enzyme cascade, SARS-CoV-2, HIV, hepatitis B, hepatitis C, luciferase, beta-galactosidase, sensitive diagnostic assays
Tags: bioluminescence technologyengineering in medicineidentifying viral particles in biological fluidsLUCAS diagnostic toolMass General Brigham researchovercoming diagnostic challengespoint-of-care testing advancementsrapid virus detection methodssensitivity in viral assaystraditional diagnostic limitationstransformative healthcare solutionsviral diagnostics innovations
What's Your Reaction?