Five years, one approval: Europe’s slow march on Alzheimer’s treatments
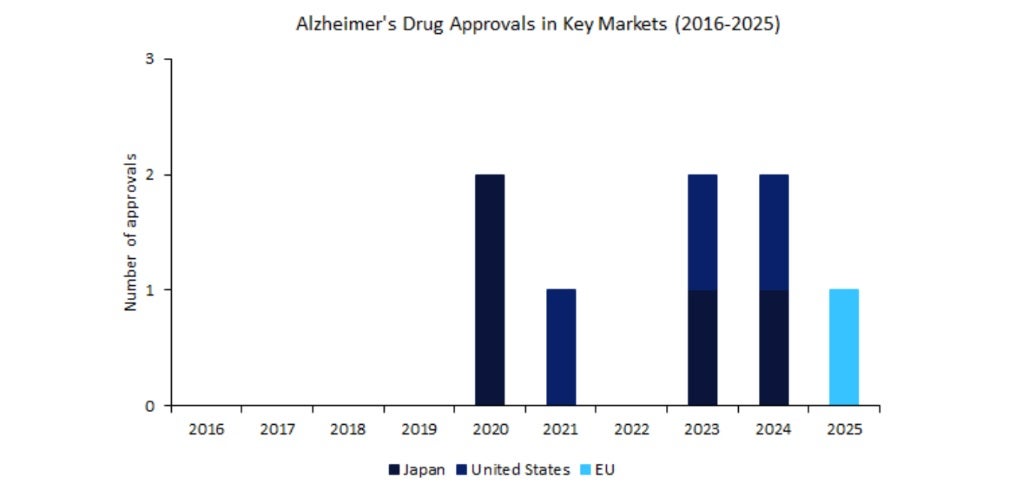
In April 2025, the European Medicines Agency (EMA) approved Eisai and Biogen’s Leqembi for early-stage Alzheimer’s disease, marking Europe’s first major nod for a disease-modifying Alzheimer’s therapy in over a decade.
What's Your Reaction?