FDA accepts Orexo’s NDA for opioid overdose medication
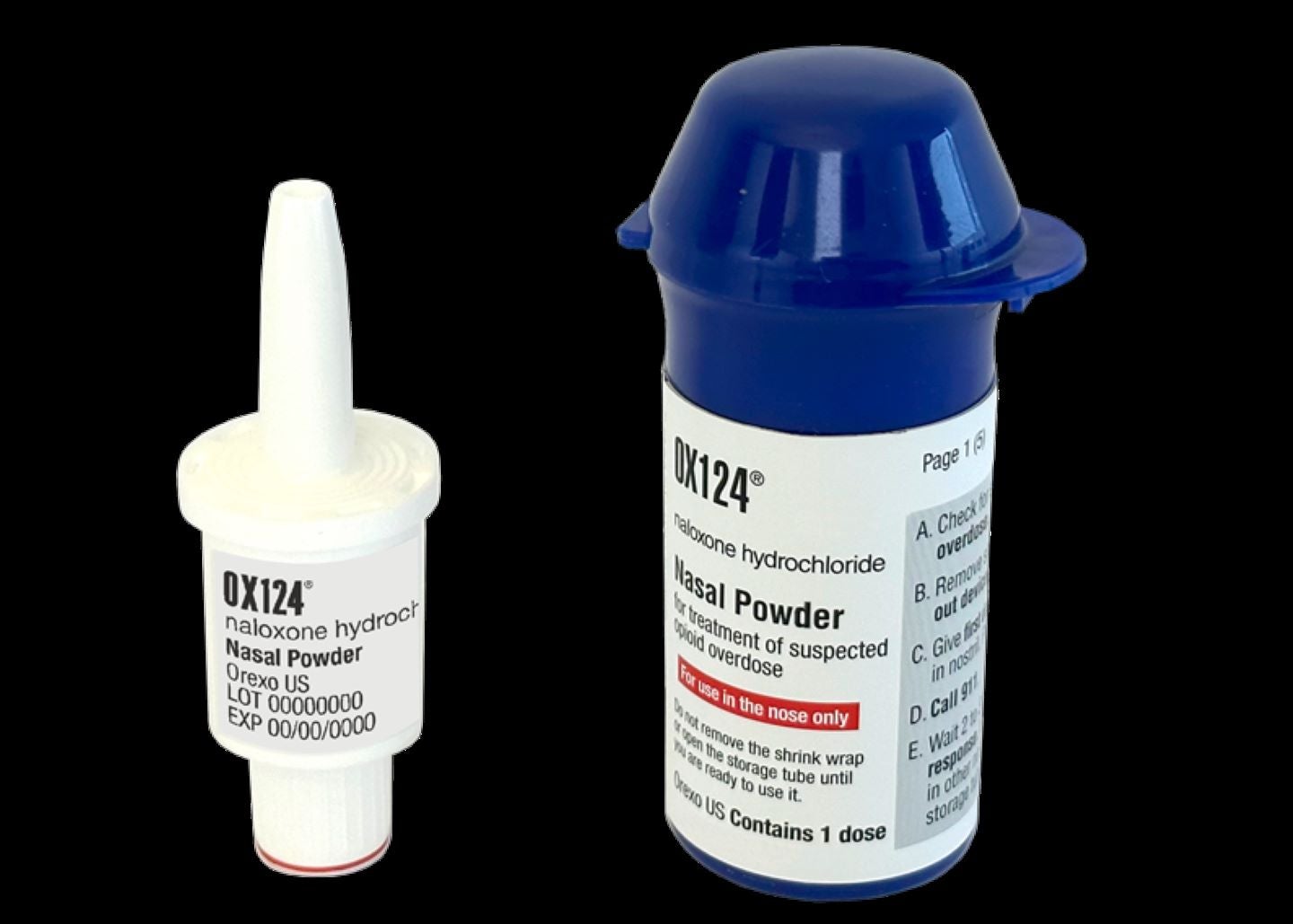
The US Food and Drug Administration (FDA) has accepted Orexo’s new drug application (NDA) for opioid overdose medication, OX124, for review. The company filed the NDA for the nasally-delivered opioid misuse rescue drug with the US regulator in September 2023. A decision is anticipated on 15 July 2024. Comprising a high dose of naloxone, OX124 leverages the company’s powder-based drug delivery approach and is designed to reverse the effects of synthetic opioids, including fentanyl. The company filed the NDA based on the data from the OX124-002 study in healthy subjects. Findings showed that OX124 offered rapid and increased naloxone absorption versus intramuscular delivery using a reference product. The drug's development formulations showed swift absorption and high bioavailability versus the market-leading naloxone rescue therapy even with the same dosage levels as the comparator in an exploratory study in healthy subjects. OX124 is patent-protected until 2039 and meets the increasing requirement for potent medicines to enhance the chances of reviving people following a drug overdose. With quick absorption and high bioavailability traits, the medication could potentially reverse an overdose or maintain consciousness in people who have taken synthetic opioids. Subject to FDA approval, the company plans to introduce it in the US in late 2024. Orexo president and CEO Nikolaj Sørensen stated: “I am pleased the FDA can now start reviewing our rescue drug, OX124. “With its high dose of naloxone and unique formulation, OX124 has the potential to reduce the acceleration in fatal overdoses caused by the widespread misuse of synthetic opioids. “With approval, we intend to initiate commercial activities during the second half of 2024 with a focus on obtaining reimbursement ahead of a broader launch into retail pharmacies early in 2025.”
What's Your Reaction?

































