Empty-handed neurons might cause neurodegenerative diseases
Tokyo, Japan – Researchers from Tokyo Metropolitan University have identified how proteins collect abnormally in neurons, a feature of neurodegenerative diseases like Alzheimer’s. They used fruit flies to show that depletion of mitochondria in axons can directly lead to protein accumulation. At the same time, significantly high amounts of a protein called eIF2β were found. […]
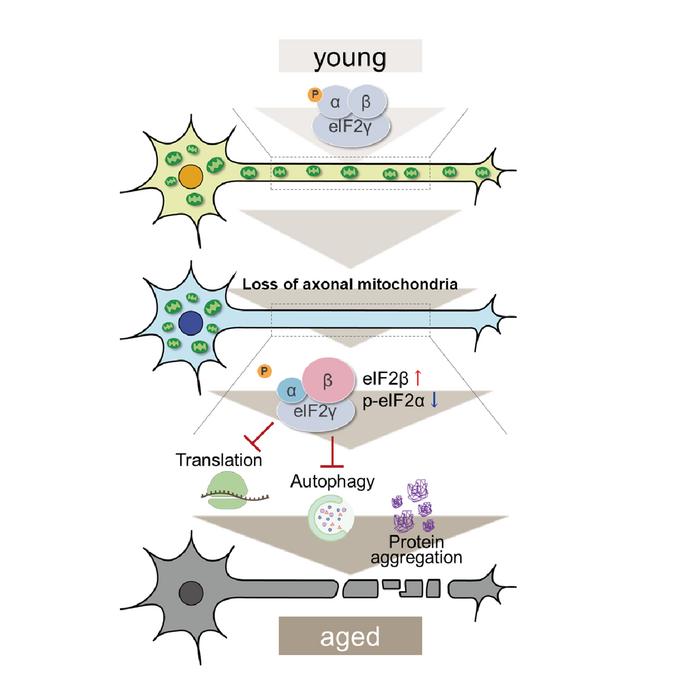
Tokyo, Japan – Researchers from Tokyo Metropolitan University have identified how proteins collect abnormally in neurons, a feature of neurodegenerative diseases like Alzheimer’s. They used fruit flies to show that depletion of mitochondria in axons can directly lead to protein accumulation. At the same time, significantly high amounts of a protein called eIF2β were found. Restoring the levels to normal led to a recovery in protein recycling. Such findings promise new treatments for neurodegenerative diseases.

Credit: Tokyo Metropolitan University
Tokyo, Japan – Researchers from Tokyo Metropolitan University have identified how proteins collect abnormally in neurons, a feature of neurodegenerative diseases like Alzheimer’s. They used fruit flies to show that depletion of mitochondria in axons can directly lead to protein accumulation. At the same time, significantly high amounts of a protein called eIF2β were found. Restoring the levels to normal led to a recovery in protein recycling. Such findings promise new treatments for neurodegenerative diseases.
Every cell in our bodies is a busy factory, where proteins are constantly being produced and disassembled. Any changes or lapses in either the production or recycling phases can lead to serious illnesses. Neurodegenerative diseases such as Alzheimer’s and Amyotrophic Lateral Sclerosis (ALS), for example, are known to be accompanied by an abnormal build-up of proteins in neurons. However, the trigger behind this accumulation remains unknown.
A team led by Associate Professor Kanae Ando of Tokyo Metropolitan University have been trying to determine the causes of abnormal protein build-up by studying Drosophila fruit flies, a commonly studied model organism that has many key similarities with human physiology. They focused on the presence of mitochondria in axons, the long tendril-like appendages that stretch out of neurons and form the necessary connections that allow signals to be transmitted inside our brains. It is known that the levels of mitochondria in axons can drop with age, and during the progress of neurodegenerative diseases.
Now, the team have discovered that the depletion of mitochondria in axons has a direct bearing on protein build-up. They used genetic modification to suppress the production of milton, a key protein in the transport of mitochondria along axons. It was found that this led to abnormal levels of protein building up in fruit fly neurons, a result of the breakdown of autophagy, the recycling of proteins in cells. Through proteomic analysis, they were able to identify a significant upregulation in eIF2β, a key subunit of the eIF2 protein complex responsible for the initiation of protein production (or translation). The eIF2α subunit was also found to be chemically modified. Both of these issues hamper the healthy action of eIF2.
Importantly, by artificially suppressing levels of eIF2β, the team discovered that they could restore the autophagy that was lost and regain some of the neuron function that was impaired due to axonal mitochondria loss. This not only shows that depletion of mitochondria in axons can cause abnormal protein accumulation, but that this happens via upregulation of eIF2β.
As populations age and the prevalence of neurodegenerative conditions continues to increase, the team’s findings present a vital step in developing therapies to combat these serious illnesses.
This work was supported by a Sasakawa Scientific Research Grant (2021-4087), the Takeda Science Foundation, a Hoansha Foundation Grant, a research award from the Japan Foundation for Aging and Health and the Novartis Foundation (Japan) for the Promotion of Science, a Grant-in-Aid for Scientific Research on Challenging Research (Exploratory) [JSPS KAKENHI Grant Number 19K21593], NIG-JOINT (National Institute of Genetics, 71A2018, 25A2019), and the TMU Strategic Research Fund for Social Engagement.
Journal
eLife
DOI
10.7554/eLife.95576.1
Article Title
Axonal distribution of mitochondria maintains neuronal autophagy during aging via eIF2β
Article Publication Date
25-Mar-2024
What's Your Reaction?

































