Continuous non-invasive glucose sensing on the horizon with the development of a new optical sensor.
For decades, people with diabetes have relied on finger pricks to withdraw blood or adhesive microneedles to measure and manage their glucose levels. In addition to being painful, these methods can cause itching, inflammation and infection. Credit: RMIT University For decades, people with diabetes have relied on finger pricks to withdraw blood or adhesive microneedles […]
For decades, people with diabetes have relied on finger pricks to withdraw blood or adhesive microneedles to measure and manage their glucose levels. In addition to being painful, these methods can cause itching, inflammation and infection.

Credit: RMIT University
For decades, people with diabetes have relied on finger pricks to withdraw blood or adhesive microneedles to measure and manage their glucose levels. In addition to being painful, these methods can cause itching, inflammation and infection.
Researchers at TMOS, the Australian Research Council Centre of Excellence for Transformative Meta-Optical Systems, have taken an important step towards eliminating this discomfort. Their RMIT University team has discovered new aspects of glucose’s infrared signature and have used this information to develop a miniaturised optical sensor only 5mm in diameter that could one day be used to provide continuous non-invasive glucose monitoring in diabetes management.
Non-invasive glucose sensing has been a target for almost 30 years due to its implications for pain free monitoring. Optical glucose sensing techniques have been reported; however, they require complex optical instrumentation usually found in laboratories, making them unsuitable for regular patient use.
The primary challenging facing affordable, wearable optical glucose testing has been miniaturisation and filtering out the glucose signals from water absorption peaks in the near infrared (NIR) spectrum. Essentially, it has been almost impossible to accurately differentiate between water and glucose in the blood. Until now.
In a first-of-its-kind research published in Advanced Sensor Research this week, the team has identified four infrared peaks in glucose that allow selective and sensitive identification in aqueous and biological environments. The team is keen to collaborate with academic and industry partners to continue this work and conduct pre-clinical and clinical research, which would open the door to the development of wearable optical glucose sensors.
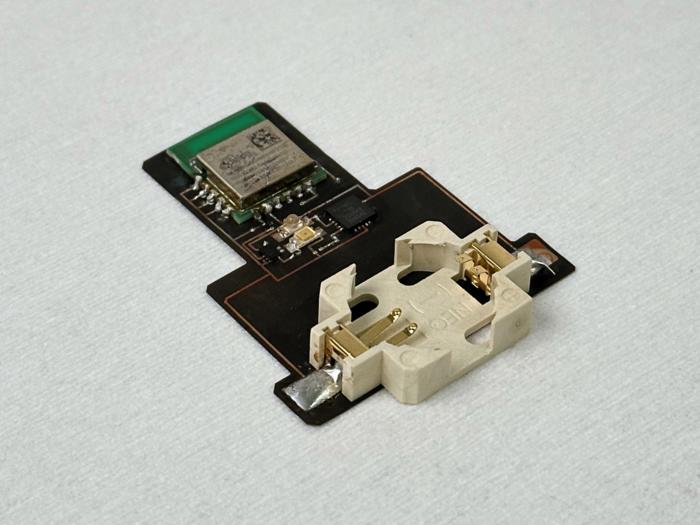
The team has fabricated a miniaturised glucose sensor established on a 1600-1700nm waveband that is Bluetooth enabled and operates using a coin battery, which allows for continuous glucose monitoring. This compact sensor has demonstrated its viability detecting glucose levels in the human body range from 50 to 400mg/dL in blood plasma, with a comparable limit of detection and sensitivity to larger, laboratory-based sensors. Its small dimensions could see it one day integrated into smart watches and other pain-free wearable health trackers.
Lead author, RMIT PhD scholar Mingjie Yang, says “Until now, there is no consensus on the unique spectroscopic signature of glucose, largely because the O-H bonds targeted in near-infrared (NIR) spectroscopy for glucose detection are also abundant in water. This similarity makes it challenging to distinguish between glucose and water signals, especially in complex biological fluids and tissues. We optimized spectroscopy setup and analysed transmittance to identify peaks unique to glucose. Our discovery finally provides the information necessary to move forward with miniaturised optical glucose sensing and we have developed a device prototype to suggest the foundation for futuristic non-invasive glucose sensor.”
The device prototype utilises a surface-mounted device light emitting diode (SMD LED) and circuits made of thin-film copper coated polymide (Cu/PI) only 110 microns thick developed with a laser patterning technology. The millimeter-scale and lightweight design of this device making it considerably more compact than traditional benchtop spectrophotometers. Furthermore, the flexible patch-like design offers the future possibility of direct reading as a wearable device on human skin.
The performance of the device has been rigorously evaluated using aqueous glucose solutions as well as in blood plasma. Computational analysis of light-skin interference has been conducted that indicate how the SMD LED will penetrate the skin. Simulation results suggest the promising locations for future exploration of optical glucose sensing in clinical setups.
TMOS Chief Investigator Madhu Bhaskaran says, “The non-invasive nature of optical glucose sensors has the potential to improve patient compliance, reduce discomfort, and lower the risks of infections associated with invasive glucose monitoring. With the right collaborators/partners and the right funding, this can represent an important shift towards continuous and pain-free glucose sensing.”
Wearable sensors, such as this one developed by TMOS researchers at RMIT, are part of Centre’s Meta Health Sensors Flagship Program—an applied research program dedicated to the development of meta-optical sensors for MedTech applications.
RMIT University has filed a patent application related to the optical glucose sensor technology that the team developed.
For more information about this research, please contact connect@tmos.org.au
Journal
Advanced Sensor Research
DOI
10.1002/adsr.202300160
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Miniaturized Optical Glucose Sensor Using 1600–1700 nm Near-Infrared Light
Article Publication Date
16-Mar-2024
What's Your Reaction?