Bioluminescence first evolved in animals at least 540 million years ago
Bioluminescence first evolved in animals at least 540 million years ago in a group of marine invertebrates called octocorals, according to the results of a new study from scientists with the Smithsonian’s National Museum of Natural History. Credit: Sönke Johnsen Bioluminescence first evolved in animals at least 540 million years ago in a group of […]
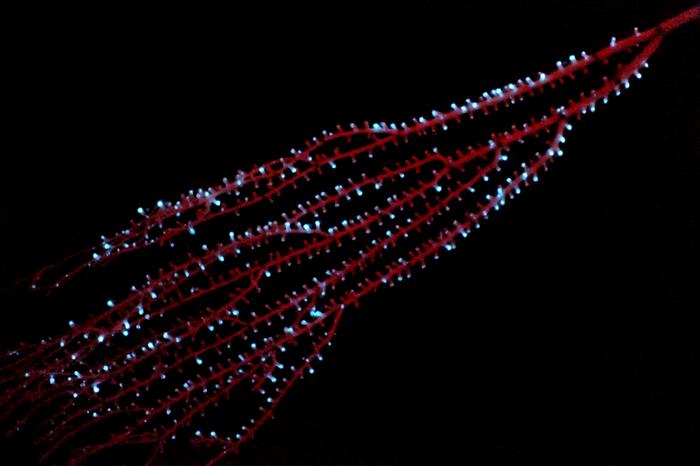
Bioluminescence first evolved in animals at least 540 million years ago in a group of marine invertebrates called octocorals, according to the results of a new study from scientists with the Smithsonian’s National Museum of Natural History.

Credit: Sönke Johnsen
Bioluminescence first evolved in animals at least 540 million years ago in a group of marine invertebrates called octocorals, according to the results of a new study from scientists with the Smithsonian’s National Museum of Natural History.
The results, published today, April 23, in the Proceedings of the Royal Society B, push back the previous record for the luminous trait’s oldest dated emergence in animals by nearly 300 million years, and could one day help scientists decode why the ability to produce light evolved in the first place.
Bioluminescence—the ability of living things to produce light via chemical reactions—has independently evolved at least 94 times in nature and is involved in a huge range of behaviors including camouflage, courtship, communication and hunting. Until now, the earliest dated origin of bioluminescence in animals was thought to be around 267 million years ago in small marine crustaceans called ostracods.
But for a trait that is literally illuminating, bioluminescence’s origins have remained shadowy.
“Nobody quite knows why it first evolved in animals,” said Andrea Quattrini, the museum’s curator of corals and senior author on the study.
But for Quattrini and lead author Danielle DeLeo, a museum research associate and former postdoctoral fellow, to eventually tackle the larger question of why bioluminescence evolved, they needed to know when the ability first appeared in animals.
In search of the trait’s earliest origins, the researchers decided to peer back into the evolutionary history of the octocorals, an evolutionarily ancient and frequently bioluminescent group of animals that includes soft corals, sea fans and sea pens. Like hard corals, octocorals are tiny colonial polyps that secrete a framework that becomes their refuge, but unlike their stony relatives, that structure is usually soft. Octocorals that glow typically only do so when bumped or otherwise disturbed, leaving the precise function of their ability to produce light a bit mysterious.
“We wanted to figure out the timing of the origin of bioluminescence, and octocorals are one of the oldest groups of animals on the planet known to bioluminesce,” DeLeo said. “So, the question was when did they develop this ability?”
Not coincidentally, Quattrini and Catherine McFadden with Harvey Mudd College had completed an extremely detailed, well-supported evolutionary tree of the octocorals in 2022. Quattrini and her collaborators created this map of evolutionary relationships, or phylogeny, using genetic data from 185 species of octocorals.
With this evolutionary tree grounded in genetic evidence, DeLeo and Quattrini then situated two octocoral fossils of known ages within the tree according to their physical features. The scientists were able to use the fossils’ ages and their respective positions in the octocoral evolutionary tree to date to figure out roughly when octocoral lineages split apart to become two or more branches. Next, the team mapped out the branches of the phylogeny that featured living bioluminescent species.
With the evolutionary tree dated and the branches that contained luminous species labeled, the team then used a series of statistical techniques to perform an analysis called ancestral state reconstruction.
“If we know these species of octocorals living today are bioluminescent, we can use statistics to infer whether their ancestors were highly probable to be bioluminescent or not,” Quattrini said. “The more living species with the shared trait, the higher the probability that as you move back in time that those ancestors likely had that trait as well.”
The researchers used numerous different statistical methods for their ancestral state reconstruction, but all arrived at the same result: Some 540 million years ago, the common ancestor of all octocorals were very likely bioluminescent. That is 273 million years earlier than the glowing ostracod crustaceans that previously held the title of earliest evolution of bioluminescence in animals.
DeLeo and Quattrini said that the octocorals’ thousands of living representatives and relatively high incidence of bioluminescence suggests the trait has played a role in the group’s evolutionary success. While this further begs the question of what exactly octocorals are using bioluminescence for, the researchers said the fact that it has been retained for so long highlights how important this form of communication has become for their fitness and survival.
Now that the researchers know the common ancestor of all octocorals likely already had the ability to produce its own light, they are interested in a more thorough accounting of which of the group’s more than 3,000 living species can still light up and which have lost the trait. This could help zero in on a set of ecological circumstances that correlate with the ability to bioluminesce and potentially illuminate its function.
To this end, DeLeo said she and some of her co-authors are working on creating a genetic test to determine if an octocoral species has functional copies of the genes underlying luciferase, an enzyme involved in bioluminescence. For species of unknown luminosity, such a test would enable researchers to get an answer one way or the other more rapidly and more easily.
Aside from shedding light on the origins of bioluminescence, this study also offers evolutionary context and insight that can inform monitoring and management of these corals today. Octocorals are threatened by climate change and resource-extraction activities, particularly fishing, oil and gas extraction and spills, and more recently by marine mineral mining.
This research supports the museum’s Ocean Science Center, which aims to advance and share knowledge of the ocean with the world. DeLeo and Quattrini said there is still much more to learn before scientists can understand why the ability to produce light first evolved, and though their results place its origins deep in evolutionary time, the possibility remains that future studies will discover that bioluminescence is even more ancient.
This study includes authors affiliated with Florida International University, the Monterey Bay Aquarium Research Institute, Nagoya University, Harvey Mudd College and University of California, Santa Cruz.
The research was supported by the Smithsonian, the David and Lucile Packard Foundation, Japan Science and Technology Agency and the U.S. National Science Foundation.
About the National Museum of Natural History
The National Museum of Natural History is connecting people everywhere with Earth’s unfolding story. It is one of the most visited natural history museums in the world. Opened in 1910, the museum is dedicated to maintaining and preserving the world’s most extensive collection of natural history specimens and human artifacts. The museum is open daily, except Dec. 25, from 10 a.m. to 5:30 p.m. Admission is free. For more information, visit the museum on its website, blog, Facebook, X (formerly Twitter) and Instagram.
# # #
Journal
Proceedings of the Royal Society B Biological Sciences
DOI
10.1098/rspb.2023.2626
Method of Research
Computational simulation/modeling
Subject of Research
Animals
Article Title
Evolution of bioluminescence in Anthozoa with emphasis on Octocorallia
Article Publication Date
23-Apr-2024
COI Statement
The authors declare they have no competing interests.
What's Your Reaction?

































