Barcoded Tracing Reveals Astrocyte-Glioma Suppression
In the relentless battle against glioblastoma (GBM), one of the deadliest primary brain cancers known to medicine, researchers have unveiled a groundbreaking method to decode the complex cellular conversations occurring within the tumor microenvironment. Despite decades of research, GBM remains notoriously resistant to immune-based therapies, largely owing to the immunosuppressive nature of its surrounding cells. […]


In the relentless battle against glioblastoma (GBM), one of the deadliest primary brain cancers known to medicine, researchers have unveiled a groundbreaking method to decode the complex cellular conversations occurring within the tumor microenvironment. Despite decades of research, GBM remains notoriously resistant to immune-based therapies, largely owing to the immunosuppressive nature of its surrounding cells. This innovative approach promises to unlock new avenues for therapeutic intervention by exposing the intricate web of cellular crosstalk that shields GBM tumors from immune attack.
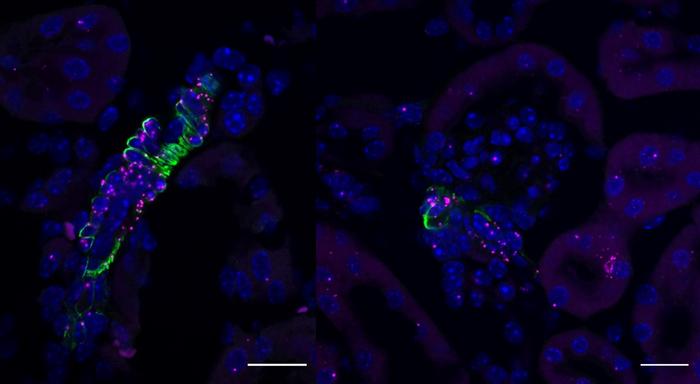
Glioblastoma’s tumor microenvironment (TME) is a dense, multifaceted ecosystem where various cell types—including immune cells, glial cells, and cancer cells—interact dynamically. Prior attempts to target GBM through immunotherapy have been stymied by the tumor’s ability to manipulate its microenvironment, effectively disarming immune responses. A deeper understanding of how these cellular players communicate was urgently needed to break this immunosuppressive barrier. Addressing this challenge, a team of scientists has pioneered a viral barcode interaction-tracing technique that enables unprecedented single-cell resolution analysis of TME interactions in human clinical samples and preclinical models.
This viral barcode method hinges on assigning unique genetic “barcodes” via engineered viruses to specific cell populations within GBM tumors. As these barcoded viruses infect different cells, their footprints can be traced through single-cell RNA sequencing, allowing researchers to map the intricate signaling pathways and physical interactions between cells. The resolution achieved through this technique surpasses traditional bulk sequencing approaches, which often mask the heterogeneity and directional cues critical to understanding cellular communication.
.adsslot_aeF95Hl7Di{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_aeF95Hl7Di{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_aeF95Hl7Di{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
By integrating this technique with comprehensive RNA sequencing datasets—both single-cell and bulk—as well as organotypic GBM cultures, the researchers could pinpoint a previously elusive bidirectional signaling axis between astrocytes, the star-shaped glial cells, and GBM tumor cells. This pathway hinges on the interaction between annexin A1 (ANXA1), a protein expressed predominantly in astrocytes, and the formyl peptide receptor 1 (FPR1), a receptor found on glioma cells. The discovery sheds light on a symbiotic communication channel that actively promotes immune evasion within the GBM microenvironment.
Functionally, FPR1 expressed on tumor cells acts as a brake on immunogenic necroptosis, a form of programmed cell death that would normally alert the immune system to cancerous threats. In parallel, ANXA1 in astrocytes suppresses key inflammatory pathways, including NF-κB signaling and inflammasome activation. Together, this dynamic reduces the immune system’s capacity to recognize and attack tumor cells effectively, reinforcing a local environment favoring tumor survival and progression.
Crucially, clinical data correlates elevated ANXA1 expression in astrocytes and high FPR1 levels in GBM cells with poorer patient outcomes, highlighting the pathway’s clinical relevance. By genetically disrupting the ANXA1–FPR1 axis through cell-specific CRISPR–Cas9 approaches in both human organ cultures and animal models, the team demonstrated a revival of the immune microenvironment. Enhanced dendritic cell, T cell, and macrophage activities were observed, accompanied by increased infiltration of tumor-specific CD8+ T cells and reduced markers of T cell exhaustion, a phenomenon that often cripples effective anti-tumor immunity.
The study’s innovative approach combining barcoded viral tracing, CRISPR-based genetic perturbation, and multiple experimental systems has set a new standard for dissecting complex TME interactions. It represents a paradigm shift from simply cataloging cellular components to understanding their precise communication networks—knowledge that is fundamental for designing next-generation immunotherapies. The identification of the ANXA1–FPR1 astrocyte–glioma signaling loop provides a compelling target whose blockade may dismantle the immunosuppressive fortress surrounding GBM.
This research not only unravels key mechanisms underlying immune evasion in glioblastoma but also signals broader implications for other solid tumors with similarly complex microenvironments. As this viral barcode tracing method gains traction, it could accelerate the discovery of hitherto hidden cellular dialogues that orchestrate tumor progression and resistance. In the wider landscape of cancer immunology, these insights bring us closer to converting immunosuppressive “cold” tumors into “hot,” immune-active ones responsive to treatment.
Beyond academic curiosity, the clinical translation of these findings may revolutionize how GBM patients are treated. Drugs targeting FPR1 or modulating ANXA1 activity could serve as adjuvants to existing immunotherapies, potentially overcoming one of the final hurdles in GBM treatment. Moreover, patient stratification based on ANXA1 and FPR1 expression levels might inform personalized therapeutic strategies, optimizing outcomes and minimizing unnecessary treatments.
The multidisciplinary approach, spanning virology, single-cell genomics, neuro-oncology, and immunology, exemplifies the power of integrative science. The use of human organotypic cultures preserves the complexity of human GBM tissue architecture, while in vivo models allow confirmation of mechanistic insights and therapeutic potential in living organisms. Together, these models provide a robust framework for translating molecular discoveries into clinical realities.
Publication of this research in a leading scientific journal underscores the profound impact of these findings. As the scientific community digests these advances, collaboration between basic scientists, clinicians, and drug developers will be critical to harness this knowledge for patient benefit. The discovery of the ANXA1–FPR1 axis stands to reshape our understanding of tumor microenvironment immunoregulation and inspire new classes of immune-modulating therapies tailored to penetrate GBM’s defensive stroma.
In sum, this study demonstrates the power of creative methodological innovation to pierce through one of cancer biology’s most intractable problems. Through barcoded viral interaction-tracing and sophisticated genetic tools, it unveils the clandestine conversation between astrocytes and glioma cells that undermines anti-tumor immunity. Such insights kindle hope that even the most formidable brain tumors may eventually be unraveled and conquered.
Subject of Research: Glioblastoma tumor microenvironment cell–cell communications; immunosuppressive astrocyte–glioma interactions; ANXA1–FPR1 signaling pathway.
Article Title: Barcoded viral tracing identifies immunosuppressive astrocyte–glioma interactions.
Article References:
Andersen, B.M., Faust Akl, C., Wheeler, M.A. et al. Barcoded viral tracing identifies immunosuppressive astrocyte–glioma interactions. Nature (2025). https://doi.org/10.1038/s41586-025-09191-9
Image Credits: AI Generated
Tags: astrocyte-glioma relationshipcancer immunology advancementscancer microenvironment dynamicscellular communication in tumorsglioblastoma researchglioblastoma treatment strategiesimmune evasion in glioblastomaimmunotherapy challenges glioblastomasingle-cell resolution analysistherapeutic interventions glioblastomatumor microenvironment interactionsviral barcode tracing technique
What's Your Reaction?